Abstract
This systematic review aimed to estimate the prevalence of use of complementary and alternative medicine (CAM) in the UK. Five databases were searched for English language, peer-reviewed surveys published between 1 January 2000 and 7 October 2011. In addition, relevant book chapters and files from our own departmental records were searched by hand. Eighty-nine surveys were included, with a total of 97,222 participants. Most studies were of poor methodological quality. Across surveys on CAM in general, the average one-year prevalence of use of CAM was 41.1% and the average lifetime prevalence was 51.8%. In methodologically sound surveys, the equivalent rates were 26.3% and 44%, respectively. In surveys with response rates >70%, average one-year prevalence was nearly threefold lower than in surveys with response rates between 21% and 50%. Herbal medicine was the most popular CAM, followed by homeopathy, aromatherapy, massage and reflexology. Many patients and consumers in the UK use CAM; healthcare professionals should therefore responsibly advise their patients about the use of CAM.
Key Words: complementary and alternative medicine, survey, systematic review
Introduction
Complementary and alternative medicine (CAM) has been defined as ‘diagnosis, treatment and/or prevention which complements mainstream medicine by contributing to a common whole, satisfying a demand not met by orthodoxy, or diversifying the conceptual framework of medicine’.1 Annual out-of-pocket expenditure in the UK on CAM has been estimated at £1.6 billion.2 Despite many assertions to the contrary, CAM is not free of a potential to cause harm, particularly as it is frequently used for serious, treatable conditions.3,4 Vis a vis such data, it would seem crucial to provide reliable data on the prevalence of its use to help prioritise a research agenda, inform policy and define educational needs.
The aim of this systematic review was to summarise and critically evaluate surveys monitoring the prevalence of use of CAM by patients and consumers in the UK during the past decade.
Methods
Systematic literature searches were performed for all English language references using AMED, CINAHL, Cochrane, Embase and MEDLINE for surveys published between 1 January 2000 and 7 October 2011. Details of the search strategy are presented in supplementary Appendix S1 (published online only). In addition, relevant book chapters, review articles and our own departmental files were searched by hand for further relevant articles. Surveys that examined the prevalence of the use of CAM by patients and consumers in the UK and provided quantitative data on prevalence were included. Surveys that only reported qualitative data were excluded. Information from the included surveys was extracted according to predefined criteria and was assessed descriptively by two independent reviewers. Any disagreements were settled through discussion. Surveys were further classified according to the following criteria: sample size, response rate and random sampling. Finally, we created a category of ‘high-quality surveys’, which had to have a sample size of >1,000, have a response rate of >70% and employ a random-sampling technique.
The following methods were considered to be CAM: acupuncture/acupressure, Alexander technique, aromatherapy, autogenic training, Ayurveda, (Bach) flower remedies, biofeedback, chelation therapy, chiropractic, Feldenkrais, herbal medicine, homeopathy, hypnotherapy, imagery, kinesiology, massage of any form, meditation, naturopathy, neural therapy, osteopathy, Qigong, reflexology, relaxation therapy, shiatsu, spiritual healing, static magnets, tai chi and yoga. Non-herbal dietary supplements and vitamins, psychotherapy, physical exercises and some physio-therapeutic modalities such as electrotherapy and ultrasound were not considered to be CAM and were therefore excluded from analyses.
We ranked the top five methods of CAM (1 = most popular) from each survey and then averaged the rank numbers across the surveys to generate an overall ranking. We also provided the total number of surveys in which a particular method of CAM was the most prevalent/popular and then calculated the averages of those figures. Where available, we calculated the average of the percentage of responders who stated that they experienced benefit or were satisfied with CAM, as well as those who reported adverse effects after using CAM and the cost for purchasing CAM.
Results
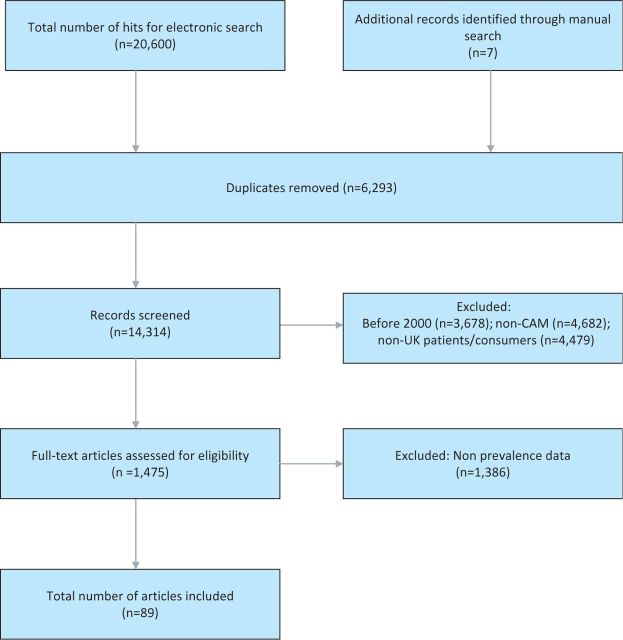
The searches generated 20,607 articles, of which 20,518 were excluded (see Fig 1). Eighty-nine surveys met our eligibility criteria.2,5–92 Detailed characteristics of the included studies are presented in Supplementary Table S1, while Supplementary Table S2 represents the surveys included on specific CAM modalities (both tables are published online only). Fifty-three surveys originated from England, 11 from Scotland, six from Wales and one from Northern Ireland. The remaining 19 surveys pertained either to the whole of the UK or their geographical location was not clearly specified.
Fig 1.
Flow diagram showing screening process. CAM = complementary and alternative medicine.
The total number of patients included in the 89 surveys was 97,222. Seventy-four surveys were on CAM in general, while the other 15 were on specific CAM modalities: 10 on herbal medicine,7,27,28,31,38,43,66,70,83,90 two on acupuncture,13,50 two on homeopathy21,22 and one on chiropractic.85 The participants included patients with asthma,26 cancer, 8,9,15,18,19,31,32,39,42,51–56,59,60,63,66,70,71,73,79 dermatological conditions,6,38,47,57 epilepsy,12 HIV,14 hypertension,77 infertility,10,13 multiple sclerosis,30,72,85 pain,16,41,49,69,84 Parkinson's disease, 75 paediatric illneses,11,23,47,51,61,64,76,87,91 surgical procedures27,62 and various other clinical conditions.2,21,50,74,82 Thirteen surveys referred to the use of CAM in healthy consumers.7,20,22,23,34,36,44,45,58,65,67,68,78
The use of a random-sampling method was mentioned in 13 (14.6%) surveys.2,7,16,20,24,26,31,34,44,55,58,64,65 The response rates ranged from 13.8% to 100% (average 69.7%). Across surveys on CAM in general, the average one-year prevalence was 41.1% (range 9.2–100%) and the average lifetime prevalence was 51.8% (range 29–71%). Across surveys on specific methods of CAM, the average one-year prevalence of use of herbal medicines was 64.2% (range 36–92.4%) and the lifetime prevalence of use of homeopathy was 70% (range 70–70%).
Perceived effectiveness of CAM was mentioned in 41 (50.5%) surveys.2,5,6,10,11,15,19,21,23,25,26,31,33,34,37,42,45,47–49,51–57,60–63,68,72–74,76,79–82,84,86,88,89,92 The average perceived effectiveness across all surveys on CAM in general was 49.7% (range 10–100%). The incidence of AEs was reported in 11 (12.3%) surveys11,28,31,52,53,55,56,69,82,83,89 and the incidence across surveys on CAM in general amounted to 5% (range 2.1–11.8%). The costs of CAM were provided in 23 (25.8%) surveys.2,11,12,18–20,23,26,30,42,46,49,52–56,59,60,64,68,73,81 Based on four surveys, the average cost of using CAM per patient per month was £15.99 (range £8.80–28.00).2,30,42,46
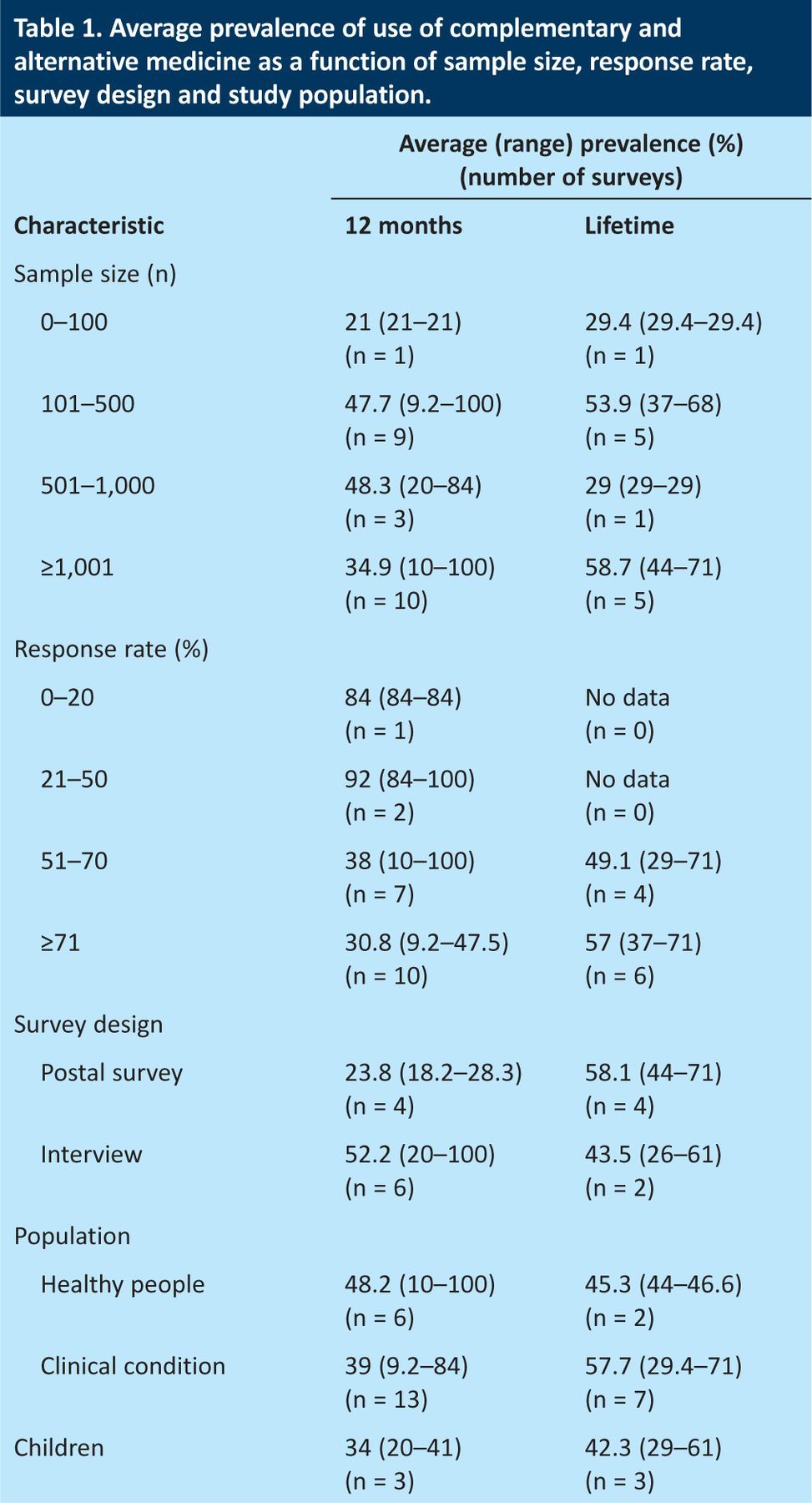
Table 1 summarises the prevalences according to sample size, response rate and survey design. In surveys with response rates of >70%, the average one-year prevalence was 30.8% (range 9.2–47.5%) and the average lifetime prevalence was 58.8% (range 37–71%). In surveys with a sample size of >1,000, the average one-year prevalence was 34.9% (range 10–100%) and the average lifetime prevalence was 58.7% (range 44–71%). In surveys with a random-sampling method, the average one-year prevalence was 21.15% (range 10–28.3%) and the average lifetime prevalence was 45.3% (range 44–46.6%). One survey met all of the above criteria for methodological acceptability44 and reported one-year prevalence of 26.3% and lifetime prevalence of 44%.
Table 1.
Average prevalence of use of complementary and alternative medicine as a function of sample size, response rate, survey design and study population.
Herbal medicine was ranked as the most popular type of CAM in 24 surveys (second in four, third in four, fourth in none and fifth in one), homeopathy in eight surveys (second in seven, third in two, fourth in two and fifth in none), aromatherapy in six surveys (second in seven, third in five, fourth in three and fifth in two), massage in six surveys (second in seven, third in two, fourth in one and fifth in none) and reflexology in four surveys (second in two, third in four, fourth in one and fifth in one) (based on surveys of use of CAM in general). Using our ranking method, herbal medicine was the most popular form of CAM (32.4% of surveys), followed by homeopathy (10.8%), aromatherapy (8.1%), massage (8.1%) and reflexology (5.4%). The average percentage values of the five most popular methods of CAM were 29.5% for herbal medicine, 37.7% for homeopathy, 24.5% for aromatherapy, 15.2% for massage and 25.4% for reflexology. Prayer and relaxation were both ranked first in 2.7% of all surveys. Acupuncture, chiropractic, osteopathy, reiki and yoga were all ranked first in 1.3% of surveys.
Discussion
Our aim was to investigate the prevalence of use of CAM in the UK by conducting a systematic review of all recent relevant surveys. Our findings are noteworthy in several respects. Firstly, the amount of surveys published in the last decade is larger than many of us would have expected. It has been noted before that ‘the number of [CAM] surveys published each year considerably and consistently exceed[s] the number of clinical trials.93 Secondly, the methodological quality of almost all of these surveys was limited.
The abundance of flawed surveys combined with the paucity of sound survey data have the potential to mislead. Depending on what article we select, we could choose almost any prevalence rate we wish. This huge variability of data on the prevalence of use of CAM has been noted repeatedly.93,94 Proponents of CAM tend to use the allegedly high prevalence of its use to argue that CAM should be made more widely available: most patients in the UK pay for CAM out of their own pocket and the argument of enthusiasts for CAM, in the name of equality and fairness, is that CAM should not just be available to those who can afford it but it should be paid for by the NHS.
A particular concern is the often low response rate in the included surveys. It seems reasonable to assume that those volunteers who fail to respond have less interest in CAM than those who do; low response rates thus might generate falsely high prevalence rates. It would be relatively easy to take this factor into account – for example, by assuming a range of prevalence rates in non-responders, which would provide a range of prevalence rates depending on such assumptions. None of the included surveys has adopted this method.
Further problems with surveys of CAM relate to the fact that no universally accepted definition of CAM exists. This means that different surveys monitor the use of different methods, which inevitably creates confusion. 93 Furthermore, none of the questionnaires used in the included surveys has been formally validated. This means that there is no certainty that they quantify what they aim to measure.
Given the abundance of problems with CAM surveys, it is difficult to trust their findings. This applies not just to the reported prevalence rates but also to all other information that such surveys might generate. For instance, we noted that the average perceived effectiveness of CAM was substantial. Assuming that proponents of CAM predominantly answer such surveys, this information is unsurprising but less than reliable. Similarly, one cannot fail to notice that the methods of CAM that are deemed to be among the most popular vary to a disconcerting extent. Considering that other evidence contradicts our list of the most popular treatments based on the included surveys, and in view of the fact that this list could be prone to frequent and rapid fluctuations, we advise caution when interpreting these data.
These and other problems seriously limit the conclusions we are able to draw despite the plethora of data. Even though many articles on CAM start with the notion that the use of CAM has been increasing, we have no sound data to confirm that this is the case in the UK. Despite the common assumption that use of CAM in the UK is high, we have little solid evidence that this is true.
In conclusion, many surveys are monitoring the prevalence of use of CAM in the UK. Due to numerous flaws and problems, the information they provide is less than reliable.
Funding
PP has a fellowship from the Royal College of Physicians (RCP), London.
Competing interest
The RCP had no role in the study design or in the data collection or interpretation.
Tables S1 and S2, and Appendix S1 can be found online at www.clinmed.rcpjournal.org.
References
- 1.Ernst E, Pittler MH, Wider B, Boddy K. The desktop guide to complementary and alternative medicine, 2nd edition Edinburgh: Elsevier Mosby, 2006. [Google Scholar]
- 2.Ernst E, White AR. The BBC survey of complementary medicine use in the UK. Complement Ther Med 2000; 8: 32–36. [PubMed] [Google Scholar]
- 3.Singh S. The hidden risks of alternative medicine. Focus Alt Complement Ther 2009; 14: 175–6. 10.1211/fact.14.3.0005 [DOI] [Google Scholar]
- 4.Hunt K, Ernst E. Patients' use of CAM: results from the Health Survey for England 2005. Focus Alt Complement Ther 2010; 15: 101–3. [Google Scholar]
- 5.Anon A. Taking an alternative route to good health. Which? 2006; Feb: 10–5. [Google Scholar]
- 6.Baron SE, Goodwin RG, Nicolaou N, et al. Use of complementary medicine among outpatients with dermatologic conditions within Yorkshire and South Wales, United Kingdom. J Am Acad Dermatol 2005; 52: 589–94. 10.1016/j.jaad.2004.11.058 [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio F, Duneclift S, Atkinson R, Cook D. Use of alternative medicines in a multi-ethnic population. Ethn Dis 2001; 11: 11–8. [PubMed] [Google Scholar]
- 8.Clare S, Buxton-King A. A pilot clinical service evaluation examining the usage and satisfaction of complementary therapy for patients treated in cancer services at University College London Hospitals NHS Foundation Trust. Abstract presented at the 36th annual meeting of the European Group for Blood and Marrow Transplantation, Vienna, Austria, 21–24 March 2010.
- 9.Corner J, Yardley J, Maher EJ, et al. Patterns of complementary and alternative medicine use among patients undergoing cancer treatment. Eur J Cancer Care (Engl) 2009; 18: 271–9. 10.1111/j.1365-2354.2007.00911.x [DOI] [PubMed] [Google Scholar]
- 10.Coulson C, Jenkins J. Complementary and alternative medicine utilisation in NHS and private clinic settings: a United Kingdom survey of 400 infertility patients. J Exp Clin Assist Reprod 2005; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford N, Cincotta D, Lim A, Powell C. A cross-sectional survey of complementary and alternative medicine use by children and adolescents attending the University Hospital of Wales. BMC Complement Altern Med 2006; 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easterford K, Clough P, Comish S, et al. The use of complementary medicines and alternative practitioners in a cohort of patients with epilepsy. Epilepsy Behav 2005; 6: 59–62. 10.1016/j.yebeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 13.Hinks J, Coulson C. An assessment of the demand and importance of acupuncture to patients of a fertility clinic during investigations and treatment. Hum Fert 2010; 13 (Suppl1):3–21. [Google Scholar]
- 14.Ladenheim D, Horn O, Werneke U, et al. Potential health risks of complementary alternative medicines in HIV patients. HIV Med 2008; 9: 653–9. 10.1111/j.1468-1293.2008.00610.x [DOI] [PubMed] [Google Scholar]
- 15.Lewith GT, Broomfield J, Prescott P. Complementary cancer care in Southampton: a survey of staff and patients. Complement Ther Med 2002; 10: 100–6. 10.1054/ctim.2002.0525 [DOI] [PubMed] [Google Scholar]
- 16.MacKichan F, Paterson C, Henley WE, Britten N. Self-care in people with long term health problems: a community based survey. BMC Fam Pract 2011; 12; 53 10.1186/1471-2296-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore AD, Petri MA, Manzi S, et al. The use of alternative medical therapies in patients with systemic lupus erythematosus. Arthritis Rheum 2000; 43: 1410–8. [DOI] [PubMed] [Google Scholar]
- 18.Needleman S, Wasim Z, Sassab S, et al. The use of complementary and alternative medicines (CAM) in brain tumor patients. Neuro-Oncology 2010; 12 (Suppl 3):iii63 (Abstract P169). [Google Scholar]
- 19.Newsom-Davis T, Kenny L, Al-Shakarchi I, et al. Voodoo dolls and the cancer patient: patients do trust their doctors. QJM 2009; 102: 311–9. 10.1093/qjmed/hcp013 [DOI] [PubMed] [Google Scholar]
- 20.Thomas KS, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England – a population based survey. Complement Ther Med 2001; 9: 2–11. [DOI] [PubMed] [Google Scholar]
- 21.Thompson E, Dahr J, Susan M, Barron S. Setting standards in homeopathic practice – a pre-audit exploring motivation and expectation for patients attending the Bristol Homeopathic Hospital. Homeopathy 2007; 96: 243–6. 10.1016/j.homp.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 22.Thompson EA, Bishop JL, Northstone K. The use of homeopathic products in childhood: data generated over 8.5 years from the Avon Longitudinal Study of Parents and Children (ALSPAC). J Altern Complement Med 2010; 16: 69–79. 10.1089/acm.2009.0007 [DOI] [PubMed] [Google Scholar]
- 23.Robinson N, Blair M, Lorenc A, et al. Complementary medicine use in multi-ethnic paediatric outpatients. Complement Ther Clin Pract 2008; 14: 17–24. 10.1016/j.ctcp.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 24.Rüdell K, Bhui K, Priebe S. Do ‘alternative’ help-seeking strategies affect primary care service use? A survey of help-seeking for mental distress. BMC Public Health 2008; 11: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakeel M, Trinidade A, Jehan S, Ah-See KW. The use of complementary and alternative medicine by patients attending a general otolaryngology clinic: can we afford to ignore it? Am J Otolaryngol 2010; 31: 252–60. 10.1016/j.amjoto.2009.02.016 [DOI] [PubMed] [Google Scholar]
- 26.Shaw A, Noble A, Salisbury C, et al. Predictors of complementary therapy use among asthma patients: results of a primary care survey. Health Soc Care Community 2008; 16: 155–64. 10.1111/j.1365-2524.2007.00738.x [DOI] [PubMed] [Google Scholar]
- 27.Skinner CM, Rangasami J. Preoperative use of herbal medicines: a patient survey. Br J Anaesth 2002; 89: 792–5. 10.1093/bja/89.5.792 [DOI] [PubMed] [Google Scholar]
- 28.Smith L, Ernst E, Ewings P, et al. Co-ingestion of herbal medicines and warfarin. Br J Gen Pract 2004; 54: 439–41. [PMC free article] [PubMed] [Google Scholar]
- 29.Smith G, Steinke D, Kinnear M, et al. A comparison of irritable bowel syndrome patients managed in primary and secondary care: the Episode IBS study. Br J Gen Pract 2004; 54: 503–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Tremlett HL, Wiles CM, Luscombe DK. Nonprescription medicine use in a multiple sclerosis clinic population. Br J Clin Pharmacol 2000; 50: 55–60. 10.1046/j.1365-2125.2000.00224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Tonder E, Herselman MG, Visser J. The prevalence of dietary-related complementary and alternative therapies and their perceived usefulness among cancer patients. J Hum Nutr Diet 2009; 22: 528–35. 10.1111/j.1365-277X.2009.00986.x [DOI] [PubMed] [Google Scholar]
- 32.Zavery B, Appleton L, Sandiford K, et al. Complementary and alternative medicine use amongst oncology patients attending a large cancer centre in England. Prog Palliat Care 2010; 18: 89–93. 10.1179/096992610X12624290276548 [DOI] [Google Scholar]
- 33.Daley A, MacArthur C, McManus R, et al. Factors associated with the use of complementary medicine and non-pharmacological interventions in symptomatic menopausal women. Climacteric 2006; 9: 336–46. 10.1080/13697130600864074 [DOI] [PubMed] [Google Scholar]
- 34.Emslie MJ, Campbell MK, Walker KA. Changes in public awareness of, attitudes to, and use of complementary therapy in North East Scotland: surveys in 1993 and 1999. Complement Ther Med 2002; 10: 148–53. 10.1016/S0965229902000663 [DOI] [PubMed] [Google Scholar]
- 35.Featherstone C, Godden D, Gault C, et al. Integrated healthcare – including complementary/alternative medicine (CAM) as an important aspect of primary care. Abstract presented at the 18th annual meting of the International Society of Technology Assessment in Health Care, Berlin, Germany, 9–12 June 2002. (Abstract 96).
- 36.Freymann H, Rennie T, Bates I, et al. Knowledge and use of complementary and alternative medicine among British undergraduate pharmacy students. Pharm World Sci 2006; 28: 13–8. 10.1007/s11096-005-2221-z [DOI] [PubMed] [Google Scholar]
- 37.Furnham A, Bond C. The perceived efficacy of homeopathy and orthodox medicine: a vignette-based study. Complement Ther Med 2000; 8: 193–201. 10.1054/ctim.2000.0381 [DOI] [PubMed] [Google Scholar]
- 38.Gee BC, Wilson P, Morris AD, Emerson RM. Herbal is not synonymous with safe. Arch Dermatol 2002; 138: 1613 10.1001/archderm.138.12.1613 [DOI] [PubMed] [Google Scholar]
- 39.Gage H, Storey L, McDowell C, et al. Integrated care: Utilisation of complementary and alternative medicine within a conventional cancer treatment centre. Complement Ther Med 2009; 17: 84–91. 10.1016/j.ctim.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 40.Greenfield S, Pattison H, Jolly K. Use of complementary and alternative medicine and self-tests by coronary heart disease patients. BMC Complement Altern Med 2008; 8: 47 10.1186/1472-6882-8-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haetzman M, Elliott AM, Smith BH, et al. Chronic pain and the use of conventional and alternative therapy. Fam Pract 2003; 20: 147–54. 10.1093/fampra/20.2.147 [DOI] [PubMed] [Google Scholar]
- 42.Harris P, Finlay IG, Cook A, et al. Complementary and alternative medicine use by patients with cancer in Wales: a cross sectional survey. Complement Ther Med 2003; 11: 249–53. 10.1016/S0965-2299(03)00126-2 [DOI] [PubMed] [Google Scholar]
- 43.Headley J, Northstone K, Simmons H, Golding J. Medication use during pregnancy: data from the Avon longitudinal study of parents and children. Eur J Clin Pharmacol 2004; 60: 355–61. 10.1007/s00228-004-0775-7 [DOI] [PubMed] [Google Scholar]
- 44.Hunt K, Coelho HF, Wider B, et al. Complementary and alternative medicine use in England: Results from a national survey. Int J Clin Pract 2010; 64: 1496–502. 10.1111/j.1742-1241.2010.02484.x [DOI] [PubMed] [Google Scholar]
- 45.Jacobs PA, Griffin JE. Women's use and interest in complementary/alternative therapies. Proc Br Psychol Soc 2001; 9: 55. [Google Scholar]
- 46.Jordan KM, Sawyer S, Coakley P, et al. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology 2004; 43: 381–4. 10.1093/rheumatology/keh045 [DOI] [PubMed] [Google Scholar]
- 47.Johnston GA, Bilbao RM, Graham-Brown RAC. The use of complementary medicine in children with atopic dermatitis in secondary care in Leicester. Br J Dermatol 2003; 149: 566–71. 10.1046/j.1365-2133.2003.05471.x [DOI] [PubMed] [Google Scholar]
- 48.Kong SC, Hurlstone DP, Pocock CY, et al. The incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol 2005; 39: 138–41. [PubMed] [Google Scholar]
- 49.Lambert TD, Morrison KE, Edwards J, Clarke CE. The use of complementary and alternative medicine by patients attending a UK headache clinic. Complement Ther Med 2010; 18: 128–34. 10.1136/jnnp.2010.226340.141 [DOI] [PubMed] [Google Scholar]
- 50.MacPherson H, Sinclair-Lian N, Thomas K. Patients seeking care from acupuncture practitioners in the UK: a national survey. Complement Ther Med 2006; 14: 20–30. 10.1016/j.ctim.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 51.Molassiotis A, Cubbin D. ‘Thinking outside the box’: complementary and alternative therapies use in paediatric oncology patients. Eur J Oncol Nurs 2004; 8: 50–60. [DOI] [PubMed] [Google Scholar]
- 52.Molassiotis A, Fernandez-Ortega P, Pud D, et al. Complementary and alternative medicine use in colorectal cancer patients in seven European countries. Complement Ther Med 2005; 13: 251–7. 10.1016/j.ctim.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 53.Molassiotis A, Fernandez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol 2005; 16: 655–63. 10.1093/annonc/mdi110 [DOI] [PubMed] [Google Scholar]
- 54.Molassiotis A, Panteli V, Patiraki E, et al. Complementary and alternative medicine use in lung cancer patients in eight European countries. Complement Ther Clin Pract 2006; 12: 34–9. 10.1016/j.ctcp.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 55.Molassiotis A, Scott JA, Kearney N, et al. Complementary and alternative medicine use in breast cancer patients in Europe. Support Care Cancer 2006; 14: 260–7. 10.1007/s00520-005-0883-7 [DOI] [PubMed] [Google Scholar]
- 56.Molassiotis A, Ozden G, Platin N, et al. Complementary and alternative medicine use in patients with head and neck cancers in Europe. Eur J Cancer Care 2006; 15: 19–24. 10.1111/j.1365-2354.2005.00615.x [DOI] [PubMed] [Google Scholar]
- 57.Nicolaou N, Johnston GA. The use of complementary medicine by patients referred to a contact dermatitis clinic. Contact Derm 2004; 51: 30–3. 10.1111/j.0105-1873.2004.00391.x [DOI] [PubMed] [Google Scholar]
- 58.Ong CK, Petersen S, Bodeker GC, Stewart-Brown S. Health status of people using complementary and alternative medical practitioner services in 4 English counties. Am J Public Health 2002; 92: 1653–6. 10.2105/AJPH.92.10.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rees R, Feigel I, Vickers A, et al. Prevalence of complementary therapy use by women with breast cancer: a population-based survey. Eur J Cancer 2000; 36: 1359–64. [DOI] [PubMed] [Google Scholar]
- 60.Scott JA, Kearney N, Hummerston S, Molassiotis A. Use of complementary and alternative medicine in patients with cancer: a UK survey. Eur J Oncol Nurs 2005; 9: 131–7. 10.1016/j.ejon.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 61.Shakeel M, Little SA, Bruce J, Ah-See KW. Use of complementary and alternative medicine in pediatric otolaryngology patients attending a tertiary hospital in the UK. Int J Pediatr Otorhinolaryngol 2007; 71: 1725–30. 10.1016/j.ijporl.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 62.Shakeel M, Bruce J, Jehan S, et al. Use of complementary and alternative medicine by patients admitted to a surgical unit in Scotland. Ann R Coll Surg Engl 2008; 90: 571–6. 10.1308/003588408X301046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakeel M, Newton JR, Bruce J, Ah-See KW. Use of complementary and alternative medicine by patients attending a head and neck oncology clinic. J Laryngol Otol 2008; 122: 1360–4. 10.1017/S0022215108001904 [DOI] [PubMed] [Google Scholar]
- 64.Simpson N, Roman K. Complementary medicine use in children: extent and reasons. A population-based study. Br J Gen Pract 2001; 51: 914–6. [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas K, Coleman P. Use of complementary or alternative medicine in a general population in Great Britain. Results from the National Omnibus survey. J Public Health 2004; 26: 152–7. 10.1093/pubmed/fdh139 [DOI] [PubMed] [Google Scholar]
- 66.Werneke U, Earl J, Seydel C, et al. Potential health risks of complementary alternative medicines in cancer patients. Br J Cancer 2004; 90: 408–13. 10.1038/sj.bjc.6601560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheeler P, Hyland ME. Dispositional predictors of complementary medicine and vitamin use in students. J Health Psychol 2008; 13: 516–9. 10.1177/1359105308088522 [DOI] [PubMed] [Google Scholar]
- 68.Andrews GJ. Private complementary medicine and older people: service use and user empowerment. Ageing Soc 2002; 22: 343–68. 10.1017/S0144686X02008668 [DOI] [Google Scholar]
- 69.Artus M, Croft P, Lewis M. The use of CAM and conventional treatments among primary care consulters with chronic musculoskeletal pain. BMC Fam Pract 2007; 8: 26 10.1186/1471-2296-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Catt S, Fallowfield L, Langridge C. What non-prescription treatments do UK women with breast cancer use? Eur J Cancer Care 2006; 15: 279–85. 10.1111/j.1365-2354.2006.00652.x [DOI] [PubMed] [Google Scholar]
- 71.Cheetham PJ, Le Monnier KJ, Brewster SF. Attitudes and use of alternative therapies in UK prostate cancer patients – isn't it time we were in the know? Prostate Cancer Prostatic Dis 2001; 4: 235–41. [DOI] [PubMed] [Google Scholar]
- 72.Esmonde L, Long A. Complementary therapy use by persons with multiple sclerosis: benefits and research priorities. Complement Ther Clin Pract 2008; 14: 176–84. 10.1016/j.ctcp.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson S, Farrelly S, Low J, et al. The use of complementary therapy by men with prostate cancer in the UK. Eur J Cancer Care 2008; 17: 492–9. 10.1111/j.1365-2354.2007.00904.x [DOI] [PubMed] [Google Scholar]
- 74.Featherstone C, Godden D, Selvaraj S, et al. Characteristics associated with reported CAM use in patients attending six GP practices in the Tayside and Grampian regions of Scotland: a survey. Complement Ther Med 2003; 11: 168–76. 10.1016/S0965-2299(03)00067-0 [DOI] [PubMed] [Google Scholar]
- 75.Ferry P, Johnson M, Wallis P. Use of complementary therapies and non-prescribed medication in patients with Parkinson's disease. Postgrad Med J 2002; 78: 612–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerasimidis K, McGrogan P, Hassan K, Edwards CA. Dietary modifications, nutritional supplements and alternative medicine in paediatric patients with inflammatory bowel disease. Aliment Pharmacol Ther 2008; 27: 155–65. 10.1111/j.1365-2036.2007.03552.x [DOI] [PubMed] [Google Scholar]
- 77.Gohar F, Greenfield SM, Beevers DG, et al. Self-care and adherence to medication: a survey in the hypertension outpatient clinic. BMC Complement Altern Med 2008; 8: 4 10.1186/1472-6882-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harding S, Swait G, Johnson I, Cunliffe C. Utilisation of CAM by runners in the UK: a retrospective survey among non-elite marathon runners. Clinical Chiropractic 2009; 12: 61–6. 10.1016/j.clch.2009.08.004 [DOI] [Google Scholar]
- 79.Helpman L. Complementary/alternative medicine use among women receiving chemotherapy for ovarian cancer: a comparison of attitudes between two patient populations. Gynecologic Oncology 2010; 116 (Suppl):S108. [Google Scholar]
- 80.Gami B, Harrington K, Blake P, et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2003; 18: 987–94. 10.1046/j.1365-2036.2003.01760.x [DOI] [PubMed] [Google Scholar]
- 81.Badger F, Nolan P. Use of self-chosen therapies by depressed people in primary care. J Clin Nurs 2007; 16: 1343–52. 10.1111/j.1365-2702.2007.01769.x [DOI] [PubMed] [Google Scholar]
- 82.Sharples FMC, Van Haselen R, Fisher P. NHS patients' perspective on complementary medicine: a survey. Complement Ther Med 2003; 11: 243–8. [DOI] [PubMed] [Google Scholar]
- 83.Howard J, Anie KA, Holdcroft A, et al. Cannabis use in sickle cell disease: a questionnaire study. Br J Haematol 2005; 131: 123–8. 10.1111/j.1365-2141.2005.05723.x [DOI] [PubMed] [Google Scholar]
- 84.Quinn F, Hughes C, Baxter D. CAM in the treatment of low back pain: perception and use by people suffering low back pain. Focus Alt Complement Ther 2005; 10 (Suppl 1):25 10.1111/j.2042-7166.2005.tb00485.x [DOI] [Google Scholar]
- 85.Carson A, Swait G, Johnson I, Cunliffe C. Chiropractic care amongst people with multiple sclerosis: A survey of MS therapy centres in the UK. Clinical Chiropractic 2009: 23–7. 10.1016/j.clch.2009.03.005 [DOI] [Google Scholar]
- 86.Baguley DM, Humphriss RL, Butler K, et al. Incidence of complementary therapy use in patients undergoing vestibular assessment. J Laryngol Otol 2006; 120: 272–5. 10.1017/S0022215106000223 [DOI] [PubMed] [Google Scholar]
- 87.Cincotta DR, Crawford NW, Lim A, et al. Comparison of complementary and alternative medicine use: reasons and motivations between two tertiary children's hospitals. Arch Dis Child 2006; 91: 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Domoney CL, Vashisht A, Studd JWW. Use of complementary therapies by women attending a specialist premenstrual syndrome clinic. Gynecol Endocrinol 2003; 17: 13–8. [PubMed] [Google Scholar]
- 89.Heuschkel R, Afzal N, Wuerth A, et al. Complementary medicine use in children and young adults with inflammatory bowel disease. Am J Gastroenterol 2002; 97: 382–8. [DOI] [PubMed] [Google Scholar]
- 90.Holst L, Wright D, Haavik S, Nordeng H. The use and the user of herbal remedies during pregnancy. J Altern Complement Med 2009; 15: 787–92. 10.1089/acm.2008.0467 [DOI] [PubMed] [Google Scholar]
- 91.Lorenc A, Robinson N, Blair M, et al. Paediatric outpatients' use of CM. Focus Alt Complement Ther 2006; 11 (Suppl 1):28. [Google Scholar]
- 92.Vashisht A, Domoney CL, Cronje W, et al. Prevalence of and satisfaction with complementary therapies and hormone replacement therapy in a specialist menopause clinic. Climacteric 2001; 4: 250–6. [PubMed] [Google Scholar]
- 93.Ernst E. Prevalence surveys: to be taken with a pinch of salt. Complement Ther Clin Pract 2006; 12: 272–5. 10.1016/j.ctcp.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 94.Hall HG, Griffiths DL, McKenna LG. The use of complementary and alternative medicine by pregnant women: a literature review. Midwifery 2011; 27: 817–24. 10.1016/j.midw.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 95.Simpson N, Pearce A, Finlay F, Lenton S. The use of complementary medicine in paediatric outpatient clinics. Ambulatory Child Health 1998; 3: 356. [Google Scholar]
- 96.Swisher EM, Cohn DE, Goff BA, et al. Use of complementary and alternative medicine among women with gynaecologic cancers. Gynaecological Oncology 2002; 84: 363–367. 10.1006/gyno.2001.6515 [DOI] [PubMed] [Google Scholar]
- 97.Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States – prevalence, costs and patterns of use. N Engl J Med 1993; 328: 246–252. 10.1056/NEJM199301283280406 [DOI] [PubMed] [Google Scholar]
- 98.Nordeng H, Havnen GC. Use of herbal drugs in pregnancy: A survey among 400 Norwegian women. Pharmacoepidemiol Drug Saf 2004; 13: 371–380. 10.1002/pds.945 [DOI] [PubMed] [Google Scholar]