Abstract
Erectile dysfunction (ED) affects millions of men worldwide with implications that go far beyond sexual activity. ED is now recognised as an early marker of cardiovascular disease, diabetes mellitus (DM) and depression. The risk factors that are associated with ED (sedentary lifestyle, obesity, smoking, hypercholesterolaemia and the metabolic syndrome) are very similar to those for cardiovascular disease (CVD). Arguably, the awareness of ED as a symptomatic entity in the post-Viagra™ age is on the rise. Nevertheless, ED is commonly missed when evaluating patients in the hospital setting, either because of lack of consideration or awareness, or through simple embarrassment (of both clinician and patient). This article provides an overview of the aetiology, assessment and importance of ED and hopes to promote its consideration in day-to-day clinical practice.
Introduction
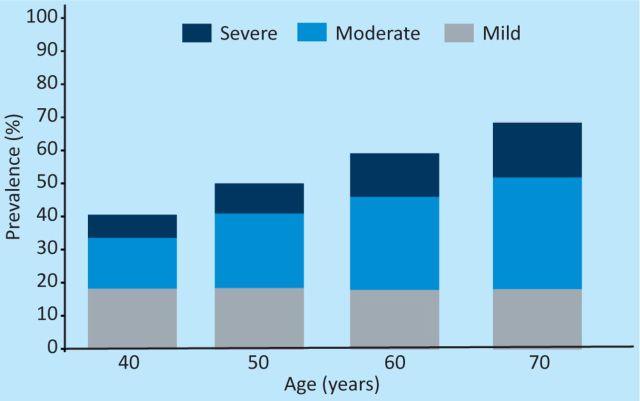
Erectile dysfunction (ED) is a common medical problem that is frequently overlooked by clinicians. The seminal Massachusetts Male Ageing study revealed that in a healthy population of men in New England aged 40–70, 52% had ED (Fig 1).1 What was especially interesting about this research was that multiple correlates were identified. These included diabetes, among other chronic diseases, as well as additional age-related modifiable phenomena such as cardiovascular risk factors.
Fig 1.
Prevalence of erectile dysfunction in men taking part in the Massachusetts male ageing study.
Inquiry into symptoms of ED should be a standard part of the evaluation of adult males presenting to clinical practice. In the same way as a menstrual history can provide valuable information about the general health of young females, ED can be a sensitive marker of systemic disease, be it metabolic, cardiovascular, neurological or otherwise. It is also likely to be of considerable importance to individual patients (having a significant psycho-social impact) and might never have been asked about before. This is especially relevant in the post-Viagra™ age in which several different treatment modalities are now available.
Evaluation of the patient with ED
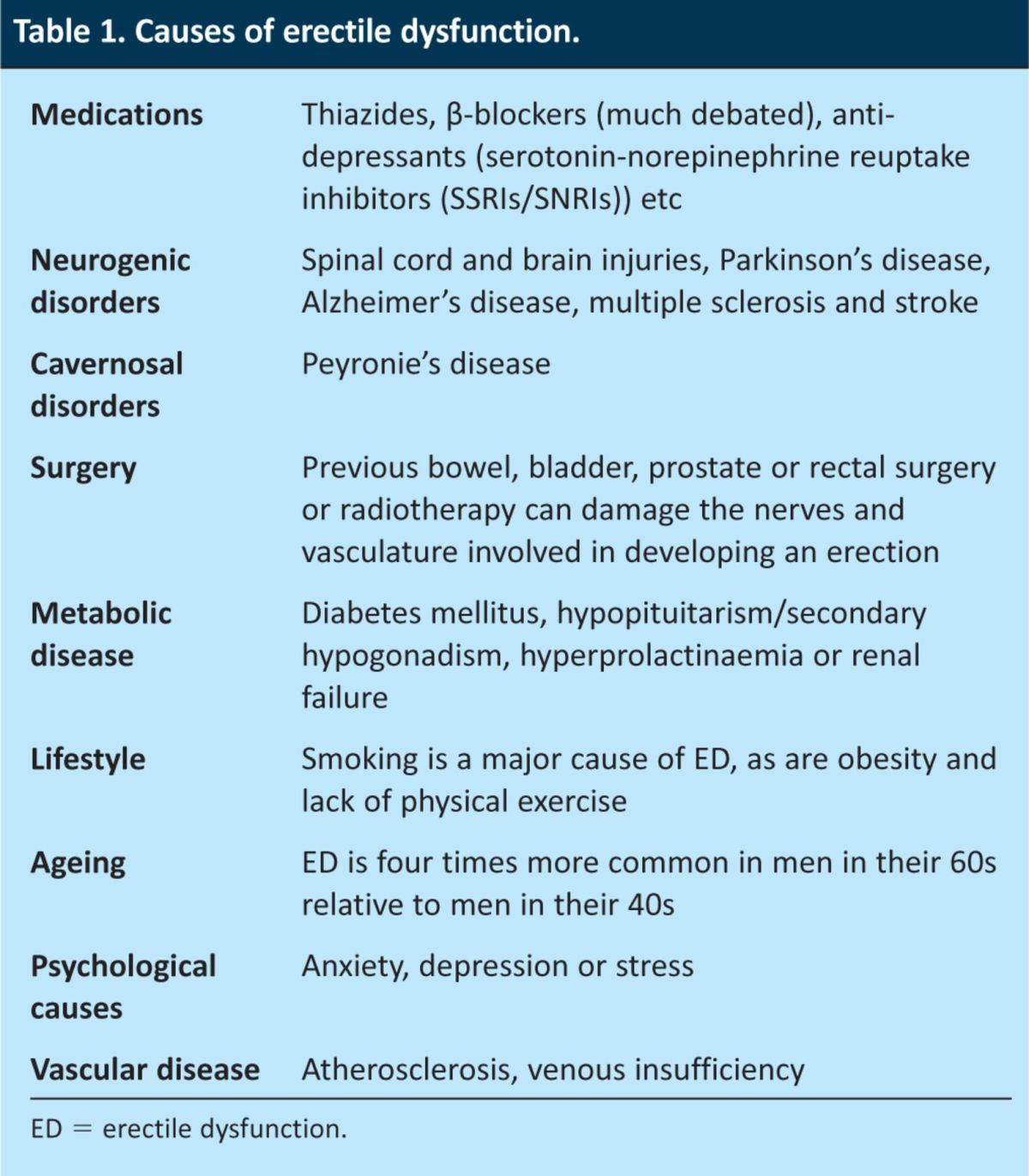
ED can emerge as part of a screen for something else entirely; for example, it is often reported when physicians enquire about the side effects of medications such as thiazides or β-blockers (ED being a well-recognised adverse effect of these drugs) or during screens for complications of other conditions such as diabetes (a specific annual enquiry about ED has now become a recommendation of NICE). A careful, sensitively taken history is key to determining the underlying cause of ED (Table 1). Such a history also allows the physician to gauge the severity of the problem and potential routes for treatment. The sexual health inventory of men (SHIM) is a useful and simple guide that the patient can use to identify the severity of the ED and it also enables the monitoring of treatment progress.
Table 1.
Causes of erectile dysfunction.
Patients can find ED to be a very difficult subject and outright, initial declaration of their problem a huge barrier to diagnosis and treatment (and this might also depend on the gender of the doctor or the patient's relationship with them).2
Factors that need to be covered include speed of onset (gradual development of symptoms usually indicates an organic cause, whereas sudden onset ED often has a psychological or traumatic cause), duration since onset, relation to the patient's libido, whether the ED is partial or complete, the circumstances under which the ED is likely to occur, and whether the patient is still able to get spontaneous morning erections (this last issue also being a good marker of testosterone status). Physical examination should focus on the patient's blood pressure, peripheral pulses, testicular size and volume, secondary sexual characteristics and any obvious anatomical defects of the penis.
Baseline investigations
Recording of cardiovascular risk factors is essential. Blood tests should include levels of gonadotrophins (luteinising hormone (LH) and follicle-stimulating hormone (FSH)), testosterone level between 9–11am (there is a marked diurnal variation in testosterone levels), sex-hormone-binding globulin level, prolactin level, fasting lipid profile and thyroid function tests. In addition, it is important to check the full blood count (testosterone has a direct effect on bone marrow and red cell production), renal function, blood glucose, liver function test and prostate-specific antigen (PSA).3
Patients should be referred to specialists if there is any obvious abnormality, be it endocrine, neurological, cardiovascular or urological.
Diabetes mellitus
Type 2 diabetes is associated with lower levels of total and free testosterone and with a higher prevalence of hypogonadism (around 25–50%) in numerous studies. This diabetic population has higher prevalence of metabolic syndrome and obesity, which are strongly associated with hypogonadism and ED. Interestingly, testosterone concentrations are inversely related to body mass index (BMI).4 Nevertheless, a significant number of non-obese patients with diabetes have subnormal testosterone levels. Consequently, this group have health-related reductions in their quality of life and are at 2–3 times greater risk of cardiovascular disease regardless of age, smoking status and low-density-lipoprotein (LDL) levels.5 By contrast, patients with type 1 diabetes have normal testosterone levels and rarely develop hypogonadism.6
The mechanism of hypogonadism in type 2 diabetes mellitus is unclear. A possible explanation relates to the effects of insulin resistance as the prevalence is higher in obese patients with diabetes. It is believed that increased aromatase enzyme activity in adipose tissue leads to increased synthesis of oestrogen, which in turn suppresses the hypothalamic secretion of gonadotropin-releasing hormone (GnRH).4 The role of insulin resistance has been demonstrated by the selective deletion of the insulin receptor gene from neurons, resulting in both a state of systemic insulin resistance and a syndrome of hypogonadotrophic hypogonadism in mice.7 Also, studies have shown a negative effect of hyperglycaemia on both the hypothalamic secretion of GnRH and the secretion of testosterone by Leydig cells, with testosterone secretion improving glycaemic control.8
Clinical features
The majority of patients with hypogonadism have non-specific symptoms such as fatigue and weakness, and around 60% of them present with ED and/or loss of libido.3 These patients are consequently at increased risk of osteoporosis, anaemia and cardiovascular disease.5 Diagnosis is confirmed by demonstrating low total and free testosterone levels together with inappropriately low LH and FSH levels. Around 5% of patients with diabetes presenting with hypogonadism have primary hypogonadism.
Evidence for treatment with testosterone
Testosterone replacement in patients with hypogonadism remains controversial. A double-blind placebo controlled study using long-acting testosterone undeconate and involving seven UK general practices showed small changes in glycated haemoglobin (HbA1c) (reduced from 7.61% to 7.52%) over 30 weeks but marked changes after a further 22 weeks (down from 8.3% to 7.3%). Mean weight loss was 2 kg with a 1.5 cm reduction in waist circumference.9 There was no significant increase in mean PSA and mean haematocrit was unchanged. Another meta-analysis showed significant reduction in fasting plasma glucose, HbA1c, fat mass and triglycerides, but no significant change in total and high-density lipoprotein cholesterol, blood pressure or BMI.10
The TIMES2 trial conducted using transdermal testosterone demonstrated a 15.2% reduction in insulin resistance (as measured by homeostasis model assessment of insulin resistance) and significant reduction in total and LDL cholesterol. There was significant increase in the International Index of Erectile Function score (IIEFs) mainly because of improvement in sexual desire and intercourse satisfaction scores.11 Another UK study has shown that testosterone replacement reduced the incidence of myocardial infarction and diabetes-related complications, improved quality-adjusted life years and is a cost-effective therapy.12
Testosterone replacement is associated with polycythaemia and liver dysfunction, so long-term monitoring is required. Finally, as the prostate is an androgen-dependant organ, there are concerns that testosterone replacement might result in testosterone-induced prostate hypertrophy and cancer. Six-monthly review of testosterone and PSA is recommended, and where there are any doubts, a urologist should perform a digital rectal examination. Studies to date have not, however, confirmed any link between testosterone replacement and prostate cancer, as outlined in a recent review by Landau and colleagues.13
Hypogonadism is common in patients with type 2 diabetes, more so if ‘metabolic syndrome’ or obesity is present. The Endocrine Society recommends routine measurement of testosterone levels in patients with type 2 diabetes, but even diabetologists can be tardy in checking this.2 Studies to date have demonstrated strong benefits resulting from testosterone replacement, but this treatment should be individualised and under specialist supervision until long-term studies fully establish its benefits and risks.
Significance of ED and cardiovascular risk
ED has the same risk factors as both coronary artery disease (CAD) and generalised vascular disease.14 These include hypertension, diabetes, hyperlipidaemia, obesity, lack of physical exercise, excess alcohol intake, poor diet and psychological stress, in particular depression. As mentioned earlier, ED is now recognised as an marker of increased cardiovascular disease (CVD) that is independent of conventional risk factors.15
A meta-analysis of prospective studies evaluated the association between ED and the risk of CVD and all-cause mortality, which had been previously recognised by consensus panels.16,17 Twelve studies involving 36,744 men with no known CVD were included. ED significantly increased the risk of CVD, CAD, stroke and all-cause mortality independently of potentially confounding conventional risk factors (Table 2). Of concern is the substantially increased 10-year risk of a cardiac event in younger men with ED (>7-fold aged 20–40 years, 50-fold aged 40–50 years), for whom a focus for risk reduction is clearly needed.18,19
Table 2.
Relative risks for men with ED when compared to those with no ED.17

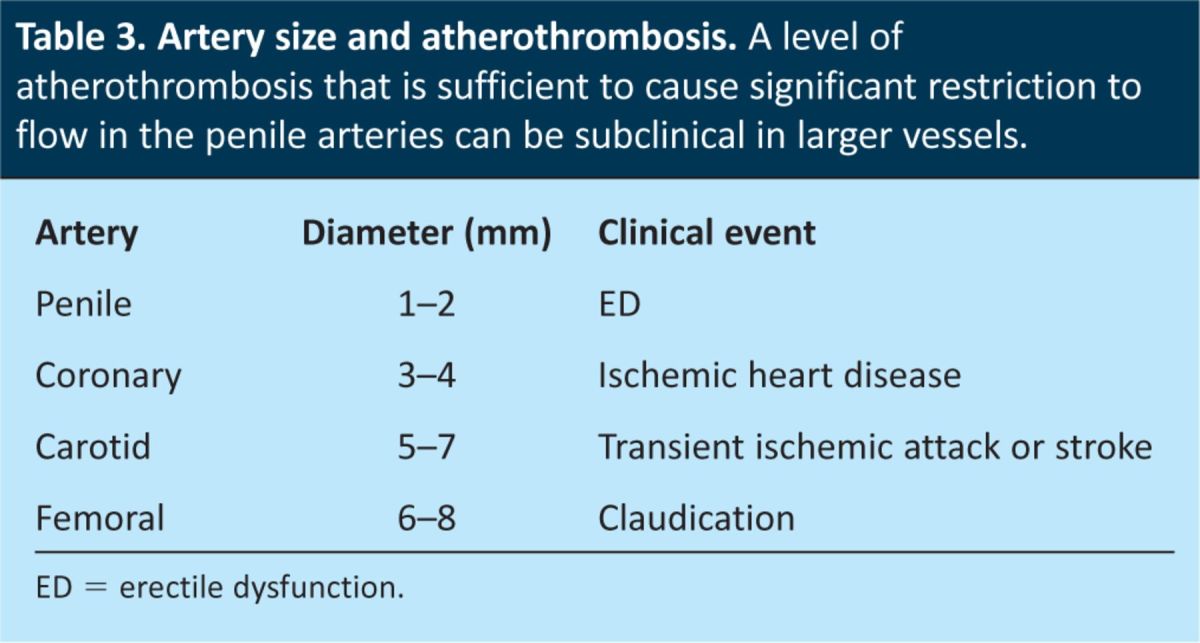
The artery size hypothesis has been used to explain how ED acts as a silent marker of vascular disease elsewhere in the body, and particularly as a marker of CAD. The lumen of the penile arteries is considerably smaller (1–2 mm) than those of the coronary (3–4 mm), carotid (5–7 mm) and femoral (6–8 mm) arteries.20 The same level of plaque burden and/or endothelial dysfunction has a greater effect on blood flow through the penile arteries than through the coronary, carotid and femoral arteries because of their smaller size (Table 3). Therefore the clinical manifestation of penile atherosclerosis, that is ED, becomes evident before the consequences of coronary (eg CAD), carotid (eg transient ischaemic attack or stroke) and femoral (eg claudication) artery atherosclerosis. The lumen of the larger arteries become significantly obstructed (>50%) only when atherosclerosis intensifies, which explains why a lot of men who present with CVD have a history of preceding ED. Furthermore, because an acute coronary syndrome often arises as the result of the rupture of a subclinical plaque, the presence of ED can also be an early warning sign of an acute event or of sudden death.
Table 3.
Artery size and atherothrombosis. A level of atherothrombosis that is sufficient to cause significant restriction to flow in the penile arteries can be subclinical in larger vessels.
In men with known CVD, in whom ED is common (prevalence 70%), ED substantially increases cardiovascular risks and is predictive of increased all-cause mortality and of the composite of cardiovascular death, myocardial infarction, stroke and admission for heart failure (hazard ratio 1.42).21
As ED and CVD frequently coexist, men with CVD should be asked about ED. Similarly, men with ED and no cardiac symptoms should be evaluated for cardiovascular risk. The time window from the onset of ED to a cardiovascular event including mortality in a man with no cardiac symptoms is two to five years, which allows time for risk-factor modification.18,19 ED should be considered a cardiovascular equivalent, and a man with ED and no cardiovascular symptoms should be considered to be a cardiovascular patient until proved otherwise. It logically follows that ED should be included in every cardiovascular risk calculator.
Lifestyle modification and aggressive management of risk factors
As ED shares modifiable risk factors with CVD, reductions in risk factors that are known to benefit CVD should in turn benefit ED. Furthermore, as ED is an independent predictor of cardiovascular events in men with no cardiac symptoms, it is now recognised to be a sentinel marker for CVD risk reduction. A recent systematic review and meta-analysis of lifestyle modification evaluated 740 men from six clinical trials, two of which included statin therapy.22 The authors concluded that an improvement in ED and sexual function results from lifestyle intervention and reduction of cardiovascular risk, and that ED gives a man the opportunity to identify cardiovascular risk factors and initiate their reduction. As physical inactivity and obesity are strongly associated with ED and CVD, they represent ideal treatment targets for improving both ED and the underlying vascular disease.
Exercise
Overall, the data suggest that increased physical activity is both a strategy to prevent the development of ED and a treatment strategy to improve erectile responses in those already suffering from ED. In a randomised controlled trial, Esposito et al performed a two-year lifestyle intervention that addressed physical activity and caloric restriction in 110 healthy obese men with ED.23 The 55 men in the intervention arm attended monthly sessions aimed at improving exercise and diet in order to achieve a 10% weight reduction, whereas those in the control group received general information regarding exercise and diet. After the two years, men in the intervention group significantly increased their physical activity, reduced their caloric intake, improved the quality of their diet and accordingly improved their erectile function from baseline, as compared with the control group. Intervention led to the complete return of erectile function in over 30% of the participants.
Obesity
Importantly, the health risk associated with obesity appears to be highly dependent on the regional deposition of adipose tissue.24 The association between anthropometric measures of abdominal obesity (eg waist circumference) and ED can be explained by excess accumulation of abdominal visceral tissue, which is an independent predictor of dyslipidaemia and insulin resistance, CVD, type 2 diabetes, inflammation and the metabolic syndrome.
Smoking
In the Health Professionals Study, smoking increased the risk of developing ED by 50%.25 In the Massachusetts Male Aging Study, men who smoked at baseline increased their risk of developing moderate or total ED to 24%, compared with 14% of non-smokers (p=0.01).26 When considering overall vascular health, advice and support to enable individuals to stop smoking are essential lifestyle interventions.
Alcohol
Excessive alcohol intake increases cardiovascular risk, but there is little evidence of increased ED risk other than the acute effect of binge drinking. Men with a very high alcohol intake are, however, unlikely to participate in studies of risk association and reduction, so the data are inconclusive at present.
Mood
Depressive disorders range from mild symptoms to major depression. Core symptoms of a depressive disorder are sadness or loss of interest or pleasure in usual activities for a period of at least two weeks, accompanied by at least five of the following: sleep difficulties, fatigue, low self-esteem, guilt, psychomotor agitation or retardation, and loss of appetite. Depressive symptoms may lead to loss of libido and reduced sexual function. Therefore, it is appropriate that patients who present with ED should be screened for depression and vice versa.27,28 This depressive symptom complex can also be caused by hypogonadism, and all men with ED should have their testosterone levels checked, as has previously been highlighted.
Offering treatment
For those with ‘generalised’ ED, the therapeutic pathway (other than targeting the underlying cause) provided in concert with lifestyle modification, is as follows:
Phosphodiesterase-5 (PDE-5) inhibitors, such as sildenafil, tadalafil and vardenafil, are among the most widely used and effective drugs for the treatment of ED. They work by temporarily increasing the blood supply to the penis (Table 4).
Urethral alprostadil is a synthetic hormone that stimulates blood flow to the penis. A small pellet can be inserted into the urethra.
Alprostadil that is administered by a direct intracavernosal injection is successful in about 85% of men who do not respond to PDE-5 inhibitors.
Surgical intervention is usually recommended only if all other treatment methods have failed. It can also be used in the context of trauma or anatomical problems. Surgery might include penile implants or vascular intervention.
Psychological therapies are important as ED can be both the cause and a consequence of a variety of mental health problems. Sex therapies such as sensate focus are available on the NHS.
Table 4.
Effective use of PDE-5 inhibitors.

Local treatment pathways tend to vary and it might be that many of the treatments listed above are co-ordinated by the urologists or a dedicated ED nurse. Vacuum-assist devices can be used at any stage. After using a vacuum pump, 9 out of 10 men are usually able to have sex, irrespective of the cause of the ED.29
Conclusions
Erectile dysfunction is an extremely important problem, with implications far beyond sexual activity. ED can be both a herald marker and a consequence of several chronic disease states. Early recognition is important because of the opportunities relating to cardiovascular risk modification.
Ultimately, the problem for clinicians is one of awareness. The signs and symptoms of ED are under-reported and are not enquired about enough routinely, which means that we miss the chance to both intervene at an early stage and to make a difference to an individual's health- (and sex-)related quality of life. If we don't ask the question, we can't do anything about the problem.
Acknowledgments
Many thanks to Dr Paul McNally, Dr David Lipscomb and Derek Lington.
References
- 1.O'Donnell AB, Araujo AB, McKinlay JB. The health of normally aging men: The Massachusetts Male Aging Study (1987–2004). Exp Gerontol 2004; 39: 975–84. [DOI] [PubMed] [Google Scholar]
- 2.Grant PS, Lipscomb D. How often do we ask about erectile dysfunction in the diabetes review clinic? Development of a neuropathy screening tool. Acta Diabetol 2009; 46: 285–90. 10.1007/s00592-008-0084-1 [DOI] [PubMed] [Google Scholar]
- 3.Ghanem HM, Salonia A, Martin-Morales A. SOP: physical examination and laboratory testing for men with erectile dysfunction. J Sex Med 2012; 10; 108–10. 10.1111/j.1743-6109.2012.02734.x [DOI] [PubMed] [Google Scholar]
- 4.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33: 1186–92. 10.2337/dc09-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Boyle J, Iyengar RL, Tamler R, et al. Hypogonadism, metabolic syndrome, and 10 year cardiovascular disease risk in middle-aged men. J Sex Med 2012,9: 1743–6095. [Google Scholar]
- 6.Tomar R, Dhindsa S, Chaudhuri A, et al. Contrasting testosterone concentrations in Type 1 and Type 2 diabetes. Diabetes Care 2006; 29: 1120–2. [DOI] [PubMed] [Google Scholar]
- 7.Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000; 289: 2122–5. [DOI] [PubMed] [Google Scholar]
- 8.Knoblovits P, Suarez SM, Scaglia HE, et al. Hypothalamic-pituitary-gonadal axis function in men with type 2 diabetes mellitus. Endo Reviews 2011; 32: Meeting Abstract 0163-769X. [Google Scholar]
- 9.Hackett G. Long-acting testosterone undecanoate improved diabetes control vs. placebo in a hypogonadal population with type 2 diabetes. J Sex Med 2011; 8: 1743–6095. [Google Scholar]
- 10.Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl 2011; 34: 528–40. 10.1111/j.1365-2605.2010.01117.x [DOI] [PubMed] [Google Scholar]
- 11.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and or metabolic syndrome (TIMES2 Study). Diabetes care 2011, 34: 828–37. 10.2337/dc10-1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudet A, Gilmour L, Church N, et al. Clinical and cost effectiveness of testosterone replacement in patients with type 2 diabetes mellitus and hypogonadism. J Men's health 2010; 7: 1875–6867. 7 10.1016/j.jomh.2010.09.027 [DOI] [Google Scholar]
- 13.Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol 2012; 76: 179–81. 10.1111/j.1365-2265.2011.04233.x [DOI] [PubMed] [Google Scholar]
- 14.Jackson G. The importance of risk factor reduction in erectile dysfunction. Curr Urol Rep 2007; 8: 463–6. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz BG, Kloner RA. Clinical cardiology: physician update: erectile dysfunction and cardiovascular disease. Circulation 2011; 123: 98–101. 10.1161/CIRCULATIONAHA.110.984179 [DOI] [PubMed] [Google Scholar]
- 16.Jackson G. Erectile dysfunction and coronary disease: evaluating the link. Maturitas 2012; 72: 263–4. 10.1016/j.maturitas.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 17.Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol 2011; 58: 1378–85. [DOI] [PubMed] [Google Scholar]
- 18.Jackson G, Boon N, Eardley I, et al. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract 2010; 64: 848–57. 10.1111/j.1742-1241.2010.02410.x [DOI] [PubMed] [Google Scholar]
- 19.Inman BA, Sauver JL, Jacobson DJ, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc 2009; 84: 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montorsi P, Montorsi F, Schulman CC. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur Urol 2003; 44: 352–4. [DOI] [PubMed] [Google Scholar]
- 21.Bohm M, Baumhakel M, Teo K, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation 2010; 121: 1439–46. 10.1161/CIRCULATIONAHA.109.864199 [DOI] [PubMed] [Google Scholar]
- 22.Gupta BP, Murad MH, Clifton MM, et al. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med 2011; 171: 1797–803. 10.1001/archinternmed.2011.440 [DOI] [PubMed] [Google Scholar]
- 23.Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291: 2978–84. 10.1001/jama.291.24.2978 [DOI] [PubMed] [Google Scholar]
- 24.Karelis AD, St-Pierre DH, Conus F, et al. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89: 2569–75. 10.1210/jc.2004-0165 [DOI] [PubMed] [Google Scholar]
- 25.Bacon CG, Mittleman MA, Kawachi I, et al. A prospective study of risk factors for erectile dysfunction. J Urol 200; 176: 217–21. 10.1016/S0022-5347(06)00589-1 [DOI] [PubMed] [Google Scholar]
- 26.Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med 2000; 30: 328–38. 10.1006/pmed.2000.0643 [DOI] [PubMed] [Google Scholar]
- 27.Seidman SN, Roose SP. The relationship between depression and erectile dysfunction. Curr Psychiatry Rep 2000; 2: 201–5. 10.1007/s11920-996-0008-0 [DOI] [PubMed] [Google Scholar]
- 28.Shiri R, Koskimaki J, Tammela TL, et al. Bidirectional relationship between depression and erectile dysfunction. J Urol 2007; 177: 669–73. 10.1016/j.juro.2006.09.030 [DOI] [PubMed] [Google Scholar]
- 29.Ilsley C, Grant P. A primary care approach to erectile dysfunction. In: Grant P. (eds), Erectile dysfunction: causes, risk factors and management. Hauppauge: Nova Science Publishers, 2012. [Google Scholar]