Abstract
Opioids can induce respiratory depression by invoking a centrally mediated decrease in involuntary respiratory rate, which in severe cases can cause a decrease in oxygen saturation. If respiratory depression is opioid induced, both low respiratory rate and low oxygen saturation will be present. If this is the case, oxygenation, rousing by verbal and physical stimulation and decreasing the opioid dose should be tried first. Naloxone, an opioid antagonist, should be avoided if at all possible but, if essential, titrate slowly to respiratory function administering 20–100 µg intravenously every two minutes. If used as a bolus for a patient on long-term opioids for chronic cancer pain, then refractory pain and symptomatic opioid withdrawal can result.
Key Words: opioid, cancer pain, respiratory depression, naloxone
Introduction
Opioids are widely used in the management of cancer pain. Although they have a range of toxicities (Table 1), respiratory depression is the effect that is most concerning to physicians. In patients on chronic opioids for cancer pain, there is a need to ensure adequate oxygenation while maintaining analgesia and avoiding opioid withdrawal. Opioid-induced respiratory depression is centrally mediated. Animal and human studies, including neuroimaging, have shown that the central drive to respiration is generated in the brainstem, with carotid and aortic chemoreceptors sensing changes in blood oxygenation.1,2 When opioids induce respiratory depression, the respiratory rate is decreased (to less than 8 breaths/min) which, in severe cases, can lead to low oxygen saturation (<85%), high arterial pCO2 values, and decreased ventilatory response to hypoxia and hypercapnea.3,4 The opioid receptor antagonist naloxone is the definitive treatment for this potentially life-threatening complication. However, naloxone reverses not only the toxicity of opioids but also their analgesia and can cause opioid withdrawal (Table 2), making the management of naloxone treatment difficult in patients with cancer pain. An accurate diagnosis of opioid-induced respiratory depression and its correct management is critical to patient care.
Table 1.
Side effects of therapeutic opioids.
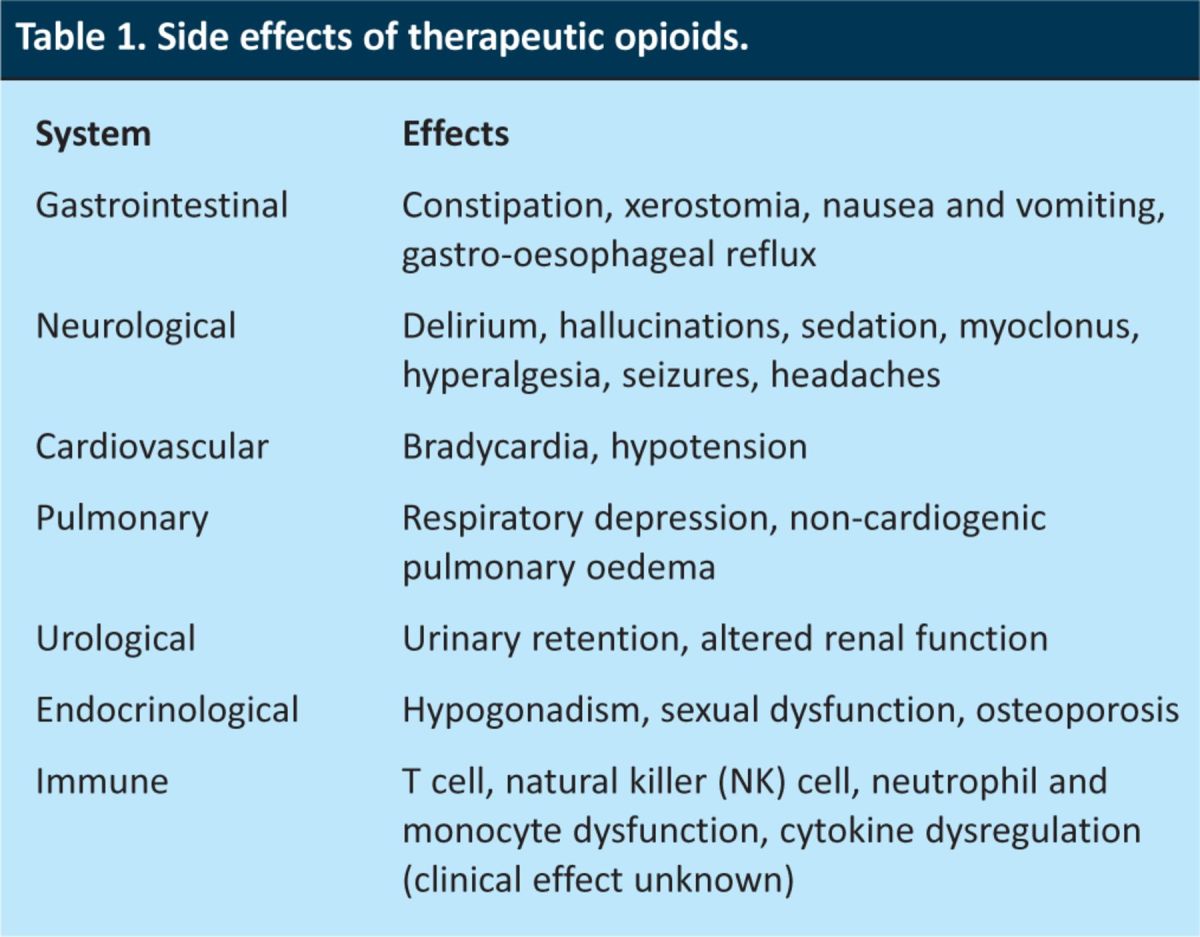
Table 2.
Effects of naloxone administration and opioid withdrawal symptoms.

Volunteer studies have illustrated that oxygen saturation is often maintained despite an opioid-induced decrease in respiratory rate. Intravenous remifentanil almost halved the respiratory rate, but decreased SpO2 by less than 3%.5 Similarly, sublingual fentanyl reduced respiratory rate (to as low as 5 breaths/min in one volunteer) but oxygen saturations never fell below 91%, and this was always manageable with breathing prompts.6 In patients receiving opioids for the relief of dyspnoea, there was no decrease in the oxygen saturation or increase in the CO2 levels.7,8 The preservation of oxygen saturations might be due to an increase in tidal volume and conservation of minute ventilation.9,10 Thus, although respiratory rate is decreased, the overall ventilation and gas exchange remains stable, as do the oxygen saturations. In severe cases of opioid-induced respiratory depression, however, this compensatory mechanism fails and the patient can develop respiratory failure with a decreased respiratory rate and a subsequent decrease in blood oxygenation.
We report two representative patients to illustrate the importance of making a correct diagnosis of opioid-induced respiratory suppression and the potential problems of using naloxone to completely reverse all the effects of the opioid, rather than just the dangerous respiratory effects.
Patients
Patient 1
A man with prostatic cancer and bone metastasis was on a stable dose of 50 mg of slow-release morphine twice daily. Two weeks following radiotherapy for hip pain, he was admitted as an emergency with a one-day history of shortness of breath and drowsiness. His respiratory rate was 41 breaths/min and his oxygen saturation was 80% on room air, this increased to 98% with 10 l/min O2. Blood gases showed a pH of 7.5, pO2 of 7.7 kPa and pCO2 of 3.7 kPa. In view of the radiotherapy, the hypoxia was thought to be caused by opioid toxicity and the patient was administered 400 µg of naloxone intravenously. This did not help his dyspnoea but increased his pain and caused opioid withdrawal symptoms. He was subsequently diagnosed with a pulmonary embolus and several days passed before pain control was regained.
Patient 2
A woman with metastatic lung cancer had been deteriorating, without a reversible cause, in hospital and was awaiting transfer to a hospice for end-of-life care. She had been on a stable dose of 15 mg/day diamorphine subcutaneously for five days. One morning, she became increasingly sleepy and her breathing became shallow with a normal respiratory rate (18 breaths/min) and oxygen saturations of 88% on 4 l/min O2. She was administered 400 µg of naloxone intravenously, which did not change her breathing rate but increased her pain level and caused agitation. She died a few hours later in pain.
Outcomes
For both patients, naloxone was administered to people on opioid therapy for cancer pain without evidence of opioid-induced respiratory depression (respiratory rate was greater than 8 breaths/min). Furthermore, naloxone was given as a bolus rather than titrating to respiratory effort, resulting in refractory reversal of the patients’ opioid analgesia and withdrawal symptoms. Although Patient 2's medical team had identified that she was approaching the end of her life and were planning for this, they still did not recognise the natural processes of dying and did not take a palliative approach.
Discussion
The only definitive indication for intravenous naloxone in patients on therapeutic opioids is respiratory depression, as evidenced by a decreased O2 saturation and respiratory rate that does not respond to verbal or physical stimulation and oxygenation. Acute reversal of opioid analgesia can be detrimental to the patient.
Optimal management plan
Confirm diagnosis
It is essential that opioid-induced respiratory depression is confirmed by observation of both a decrease in respiratory rate and impaired gas transfer with low oxygen saturation on pulse oximetry (or a low pO2 and increased pCO2 from arterial blood gases). The importance of considering other differential diagnoses for respiratory dysfunction in these cancer patients is essential. These include pneumonia, pulmonary embolism and multi-organ failure, which might present with a decrease in oxygen saturation but not a reduction in the respiratory rate; in fact, these patients are often tachypnoeic.
Conversely, a decrease in respiratory rate might not be associated with a decrease in oxygenation; hence, both reduced respiratory rate and a decrease in oxygen saturation are necessary for the diagnosis of respiratory depression.
Conservative measures
Consideration should be given as to whether the patient can be managed by conservative measures, such as rousing by verbal or physical stimulation, oxygenation (which may include bag and masking to remove CO2), and possibly decreasing the opioid dose with careful monitoring. These may improve cerebral oxygenation and the patient's level of consciousness, enabling them to breathe spontaneously more effectively and avoiding the need for naloxone. Precipitating factors such as recent opioid dose increases or renal failure need to be identified and treated and dose adjustment must be considered.
Titration of naloxone
If naloxone is needed despite conservative measures, it should be titrated on an individual basis to obtain an optimal respiratory response while maintaining adequate analgesia. As naloxone has an onset of less than two minutes after intravenous administration, it should be given at a rate of 20–100 µg iv every two minutes, titrated to respiratory function not to the pain or consciousness level (See Box 1).4,11–13 This is a very different treatment to that for opioid-induced respiratory depression in intravenous drug users, for whom the aim is to reverse all the actions of the opioids using a 400 µg bolus. The elimination half-life of naloxone is approximately 60 minutes after intravenous administration. Thus, it might need to be administered several times to patients on an opioid with a long half-life and an iv infusion of naloxone might be required. The opioid used and its dose also needs to be reviewed. If naloxone is needed by patients who are on chronic opioids for cancer pain, specialist advice should be sought regarding dose reduction and changing the opioid as, for example, buprenorphine might cause less respiratory depression than some other opioids.14,15 It is also essential to optimise the use of non-opioid analgesics.
Box 1. Administration of naloxone for use in opioid-induced respiratory depression.
In conclusion, opioid-induced respiratory depression, with low respiratory rate and low blood oxygenation, is potentially life threatening and emergency management is essential. While preserving oxygenation, the initial step of management is correct diagnosis, followed by conservative management if appropriate. If the hypoxia does not respond to this, then intravenous naloxone, titrated to respiratory effort, might be needed. Naloxone is not, however, without risk for the patient taking long-term opioids for cancer pain as it has the potential to cause opioid withdrawal and opioid refractory pain. Thus, it is vital to ensure the correct diagnosis and to try more conservative management if appropriate before using naloxone.
Competing interests
All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organisation for the submitted work; no financial relationships with any organisation that might have an interest in the submitted work; and no other relationships or activities that could appear to have influenced the submitted work. There was no additional funding.
Patient consent was not available as the patients are deceased. Cases have been anonymised to protect patient confidentiality.
Acknowledgments
The authors are grateful for assistance from Geraint H Lewis, chief data officer, NHS commissioning board; Jane Alty, neurology SpR, Leeds Teaching Hospitals NHS Trust; and Carsten Grimm, substance misuse specialist.
All authors co-wrote the manuscript. Dr Jason Boland wrote the first draft, which was modified by all authors.
References
- 1.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 2006; 7: 232–42. 10.1038/nrn1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattinson KT, Mitsis GD, Harvey AK, et al. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage 2009; 44: 295–305. 10.1016/j.neuroimage.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 3.NHS Scotland. Palliative care guidelines — naloxone, 2009. www.palliativecareguidelines.scot.nhs.uk/pain_management/naloxone.asp [Accessed 29 January 2013].
- 4.Twycross R, Wilcock A. Palliative care formulary. 4th ed. Nottingham: Palliativedrugs, 2011. [Google Scholar]
- 5.Cronin AJ, Aucutt-Walter NM, Budinetz T, et al. Low-dose remifentanil infusion does not impair natural killer cell function in healthy volunteers. Br J Anaesth 2003; 91: 805–9. 10.1093/bja/aeg273 [DOI] [PubMed] [Google Scholar]
- 6.Lister N, Warrington S, Boyce M, et al. Pharmacokinetics, safety, and tolerability of ascending doses of sublingual fentanyl, with and without naltrexone, in Japanese subjects. J Clin Pharmacol 2011; 51: 1195–204. 10.1177/0091270010379410 [DOI] [PubMed] [Google Scholar]
- 7.Clemens KE, Klaschik E. Symptomatic therapy of dyspnea with strong opioids and its effect on ventilation in palliative care patients. J Pain Symptom Manage 2007; 33: 473–81. 10.1016/j.jpainsymman.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 8.Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naive palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11: 204–16. 10.1089/jpm.2007.0131 [DOI] [PubMed] [Google Scholar]
- 9.Bouillon T, Bruhn J, Roepcke H, Hoeft A. Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesthesiol 2003; 20: 127–33. [DOI] [PubMed] [Google Scholar]
- 10.Barbour SJ, Vandebeek CA, Ansermino JM. Increased tidal volume variability in children is a better marker of opioid-induced respiratory depression than decreased respiratory rate. J Clin Monit Comput 2004; 18: 171–8. 10.1023/B:JOCM.0000042922.63647.b9 [DOI] [PubMed] [Google Scholar]
- 11.Electronic Medicines Compendium. Summary of Product Characteristics – Naloxone, 2008. www.medicines.org.uk/EMC/medicine/21095/SPC/Naloxone+400+micrograms+ml+solution+for+Injection+(hameln) [Accessed 29 January 2013].
- 12.American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. Glenview, Illinois: American Pain Society, 2008. [Google Scholar]
- 13.NHS Lothian Joint Formulary. Section 15.1.7. Antagonists for central and respiratory depression, 2011. www.ljf.scot.nhs.uk/LothianJointFormularies/Adult/15.0/15.1/15.1.7/Pages/default.aspx [Accessed 29 January 2013].
- 14.Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 2005; 94: 825–34. 10.1093/bja/aei145 [DOI] [PubMed] [Google Scholar]
- 15.Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96: 627–32. 10.1093/bja/ael051 [DOI] [PubMed] [Google Scholar]



