Abstract
Endocrine disease is common in pregnancy. Most pre-existing endocrine conditions, if well controlled, have little impact on maternal or fetal morbidity. Uncontrolled endocrine conditions in pregnancy, whether poorly controlled pre-conception or newly diagnosed, are associated with a variety of adverse fetal outcomes and maternal morbidity. Also, transplacental transfer of maternal antibodies can have adverse fetal or neonatal consequences. The initial diagnosis of many conditions is hindered by the overlap of symptoms that occur in normal pregnancy and those that suggest specific endocrine pathologies, and also by the changes in reference ranges for common biochemical measurements that occur as a result of physiological changes in pregnancy. This article summarises the common endocrine disorders in pregnancy and describes how pregnancy can alter their investigation, treatment and ongoing management, as well as the potential effects on the fetus.
Introduction
The extensive hormonal adaptations that are necessary in pregnancy occur as a result of interactions between the fetoplacental unit and the maternal endocrine system. These adaptations are extensive and are crucial for pregnancy, delivery and breastfeeding to occur successfully. Almost every endocrine axis is altered in pregnancy; therefore, the biochemical tests used to assess endocrine function must be interpreted with caution. The changes that occur in pregnancy can also alter the clinical progression of endocrine abnormalities (for example prolactinomas), so additional monitoring might be required during the antenatal and postnatal period.
Pituitary disease
Prolactinomas
Prolactinomas are the most common pituitary tumours seen in women of child-bearing age.1 Hyperprolactinaemia is a common cause of infertility in women as it inhibits the release of pulsatile gonadotrophin-releasing hormone from the hypothalamus. Treatment with a dopamine agonist can result in the rapid return of fertility and, therefore, an improved probability of conception.
In normal pregnancy, the prolactin concentration in the blood increases, so measurements of prolactin level are rarely of value in the diagnosis or management of a prolactinoma. The pituitary increases in size in normal pregnancy just as a prolactinoma might also increase in size. This growth is seen more often for macroprolactinomas (>10 mm in size) than for microprolactinomas (<10 mm) and the risk is greatest in the third trimester. The risk of tumour growth is reduced if the prolactinoma has been identified and treated before conception. Visual field testing should be performed in each trimester and pituitary imaging is required if tumour enlargement is suspected from the symptoms reported or from changes in visual fields.
Dopamine receptor antagonists are usually discontinued in early pregnancy, but can be continued in patients who have macroprolactinomas and who are at risk of symptomatic tumour expansion. If there is confirmed evidence of tumour enlargement, these drugs can be restarted and continued by lactating mothers with no detrimental effect on the neonate, but they can interfere with breastfeeding. It is important to be aware that the British Medicine and Healthcare Products Regulatory Authority has advised that pregnancy should be excluded before ergot derivatives (cabergoline and bromocriptine) are used because of a theoretical risk of maternal or fetal cardiac valve fibrosis. At present, however, there are no studies to support this advice.
Acromegaly
Pregnancy in patients with acromegaly is uncommon because the majority undergo curative surgery. Those that do not typically have problems conceiving as a result of hyperprolactinaemia from pituitary stalk compression. Most diagnostic assays cannot distinguish between pituitary and placental growth hormone, so confirmation of a diagnosis of acromegaly might have to wait until after delivery, when levels of placental growth hormone fall rapidly. Insulin-like growth factor is also present in greater concentrations in normal pregnancy.
Acromegaly can increase the risk of gestational diabetes and pregnancy-induced hypertension. The associated cardiac conditions, including coronary artery disease and cardiomyopathy, can manifest for the first time in pregnancy.
Medical treatment is usually interrupted at the time of pregnancy. Dopamine receptor antagonists are normally stopped early in pregnancy and somatostatin analogues withheld due to a lack of clear safety data surrounding their use. Interruption for the duration of pregnancy will not usually affect the course of the condition but should be decided upon on a case-by-case basis.
Only a small number of women with acromegaly have been reported to have had this condition worsen in pregnancy, but there have been a few reports of tumour growth and one case of pituitary apoplexy. Therefore, the advice given for prolactinomas relating to tumour sizing and monitoring should also be followed for pregnant women with acromegaly.
Pituitary insufficiency
Pituitary insufficiency can result from previous pituitary surgery or radiotherapy, lesions such as adenomas, infarction or lymphocytic hypophysitis. It can lead to subfertility as the gonadotrophin stimulus to ovulation might be absent, and hence ovulation-induction therapies might be required. If adequate hormonal replacement has been achieved prior to pregnancy, this condition has no effect on maternal or fetal outcome. If the condition is not diagnosed or is inadequately treated, it can be associated with miscarriage and stillbirth.2
Lymphocytic hypophysitis
Lymphocytic hypophysitis occurs at increased frequency in late pregnancy and postpartum. In contrast to pituitary insufficiency from most other causes, lymphocytic hypophysitis can cause isolated adrenocorticotropic hormone (ACTH) deficiency.
Diagnosis in the acute setting is challenging because the hormone levels used to diagnose pituitary insufficiency are affected by pregnancy. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are suppressed, so the diagnosis is limited to assessment of the thyroid and corticoadrenal axis. Thyroid function might be normal initially due to the long half-life of thyroxine (T4), so repeated measurements are important. Assessment of the corticoadrenal axis relies on ACTH and cortisol levels. The adrenal response to ACTH will remain normal in acute pituitary insufficiency so an ACTH stimulation test is not diagnostically useful. Prolactin can be useful as its level may be abnormally low.
Diabetes insipidus
The symptoms of diabetes insipidus can worsen in pregnancy as a result of placental vasopressinase production and an associated reduction in antidiuretic hormone (ADH) levels. ADH analogues such as desmopressin can be continued and no adverse effects have been reported, but a higher-than-normal dose might be required by pregnant women. It is important to reduce the dose rapidly after delivery.
Transient diabetes insipidus can occur in pregnancy secondary to placental vasopressinase production. This can be a feature of significant hepatic pathology, such as acute fatty liver of pregnancy. In this condition, the hepatic breakdown of placental vasopressinase is reduced. It is therefore important to exclude liver disease as well as other pituitary disorders that can initiate diabetes insipidus in pregnancy.
As diabetes insipidus is a disorder of the posterior pituitary, it can be associated with reduced oxytocin production. This can have important consequences at delivery, as labour may not progress and there is an increased rate of uterine atony.
Adrenal disease
Cushing's syndrome
Previously treated Cushing's syndrome with complete resolution does not alter the course of pregnancy.
The clinical features of hypercortisolaemia, for example weight gain, fatigue, glucose intolerance and hypertension, overlap with those of pregnancy. More specific features that suggest hypercortisolaemia are red or purple striae (compared to pale striae in pregnancy), proximal myopathy, fractures related to underlying osteopenia or osteoporosis, and features of hyperandrogenism including hirsuitism or acne.
In pregnancy, most cases of Cushing's syndrome result from adrenal adenomas (with a small number from adrenal carcinomas) rather than from pituitary adenomas. This could be a consequence of the reduced fertility of women with pituitary tumours.
Pregnancy affects both plasma and urinary cortisol concentrations, but failure to suppress cortisol production as measured by a high-dose dexamethasone suppression test is consistent with adrenal hypercortisolaemia. Imaging can be performed to identify the adrenal or pituitary source.
Greater incidences of pre-eclampsia, gestational diabetes mellitus, fetal loss and preterm delivery are associated with Cushing's syndrome. Neonatal adrenal insufficiency can occur because fetal cortisol production is suppressed in utero by the high circulating levels of maternal cortisol.
In women who have Cushing's syndrome, there is an improved live birth rate for those whose treatment was initiated before 20 weeks’ gestation.3 Treatment in pregnancy is therefore advocated for pregnant women who are diagnosed as having Cushing's syndrome. Surgical treatment should be considered and there are reports of successful transphenoidal resection of pituitary adenomas and laparoscopic adrenalectomies during pregnancy. Medical treatment can be used but is less effective. Metyrapone has been used, but severe hypertension has been associated with its use. Ketoconazole is avoided in pregnancy as it is teratogenic in animal studies.
Conn's syndrome
Primary hyperaldosteronism is rarely reported in pregnancy. This probably reflects under-diagnosis given the incidence of this condition in the non-pregnant population. Hypokalaemia is described as a classic feature of this syndrome, but in fact it is only seen in up to 40% of non-pregnant individuals with Conn's.4 The incidence of hypokalaemia associated with Conn's syndrome in pregnancy might be less than this because of the competitive inhibition by progesterone of sodium–potassium exchange in the distal renal tubule. The reported exacerbation of primary hyperaldosteronism in the postpartum period is likely to be explained by loss of this progesterone-mediated effect.
Plasma aldosterone concentration increases in pregnancy and the normal range overlaps with that indicating pathology in Conn's syndrome in non-pregnant individuals.5 The diagnosis can be made, however, by demonstrating a suppressed level of renin activity in the presence of raised aldosterone. Imaging of the adrenals can be performed, but if this does not identify an adenoma and/or biochemical testing is equivocal, further investigation can be postponed until after delivery.
Hyperaldosteronism can increase the rate of hypertension and proteinuria in pregnancy, with a corresponding increase in placental abruption and preterm delivery.
The use of spironolactone is cautioned in pregnancy because of its potent anti-androgen effects, which may affect the masculinisation of a male fetus. Its use could however be considered after a fetus has been confirmed to be female. Amiloride is an alternative potassium-sparing agent that can be used.
Adrenal insufficiency
In patients with known adrenal insufficiency, glucocorticoid and mineralocorticoid replacement can be continued without dose adjustment in pregnancy, except for patients who are experiencing stress such as vomiting in the first trimester or concurrent infection, who require an increased glucocorticoid dose. Stress doses of parenteral glucocorticoid should be given at the time of delivery. This advice should also be followed for patients who are at risk of iatrogenic adrenal suppression as a result of long-term steroid replacement.
First diagnosis of adrenal insufficiency in pregnancy is challenging as many of the clinical features of hypoadrenalism occur in pregnancy, such as nausea, vomiting, weakness and hyponatraemia. Nevertheless, weight loss, hyperpigmentation in skin folds, hyponatraemia of greater magnitude than that expected in pregnancy (>5 mmol/l reduction) or hypoglycaemia are not features of normal pregnancy and should be investigated further.
A short synacthen test that would routinely be performed outside pregnancy can be performed, but a normal response might not reflect normal adrenal function. The changes in cortisol-binding globulin concentration and the adrenal response to ACTH that occur particularly in the latter stages of pregnancy will affect the interpretation of any tests that rely on the measurement of total rather than free cortisol.6,7 Cortisol concentrations that are abnormally low for women who are pregnant could therefore fall within the normal range for non-pregnant individuals. If there is clinical concern despite a stimulation test result that falls within the normal range, then it is reasonable to treat with replacement glucocorticoids and perform confirmatory tests after delivery.
Adrenal antibodies might aid the identification of autoimmune adrenal insufficiency but their absence does not exclude the diagnosis. These antibodies cross the placenta but rarely result in neonatal disease.
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) is caused by a group of disorders featuring enzymatic defects in adrenal steroid synthesis, the most common types being 21-hydroxylase deficiency and 11β-hydroxylase deficiency. The phenotype can vary, from severe virilisation or salt wasting that presents in the neonatal period to non-classic CAH presenting in adulthood with hirsuitism and menstrual irregularity.
Patients with salt wasting are dependent on exogenous glucocorticoid and aldosterone replacement and, like those whose adrenal insufficiency has a different cause, might require parenteral steroid supplementation at times of stress.
CAH is associated with subfertility.8,9 Delivery can be complicated by cephalopelvic disproportion, which can result from the android pelvis and can be affected by previous genital surgery. Women with CAH are also likely to be at greater risk of pregnancy-induced hypertension and gestational diabetes mellitus.10
In utero, there is a risk of virilisation if the fetus is female and is homozygous for the recessive gene, a risk that occurs only if the partner of a woman with CAH is also a carrier. Administration of steroids such as dexamethasone can suppress fetal androgen production and ideally this is started prior to nine weeks of gestation.10 However, empirical treatment of all mothers with CAH would mean that the majority of fetuses (male fetuses and unaffected female fetuses) would be subject to unnecessary steroid exposure. Furthermore, the steroid dose required could cause mothers to become cushingoid. Therefore, a decision about steroid replacement should be made after establishing the partner's carrier status and after consideration of the likely severity of disease in the fetus. If treatment is given, the need for ongoing glucocorticoid therapy should be evaluated using ultrasound or genetic tests to determine the fetal sex, and subsequent chorionic villus sampling or amniocentesis to confirm the fetal genotype.
Phaeochromocytoma
Hypertension resulting from phaeochromocytomas can be mistaken for hypertensive disorders of pregnancy. Unrecognised phaeochromocytomas have a mortality of up to 50% at the time of labour or during induction of general anaesthesia.12
A growing, gravid uterus can impact on phaeochromocytomas, in particular those occurring at extra-adrenal locations which might be more influenced by changes in position or by uterine contractions. Physical compression can cause haemorrhage into the tumour or precipitate a hypertensive crisis. The catecholamines produced can cause uteroplacental vasoconstriction and placental insufficiency or abruption. The fetal mortality is about 26% in undiagnosed cases and 11% in diagnosed cases.
The investigation and treatment of phaeochromocytomas is not affected by pregnancy. After detection of abnormal urinary catecholamines and imaging, alpha then beta receptor blockade should be commenced. Surgical resection can be performed prior to 23 weeks of gestation if sufficient pharmacological blockade has been established, but can be delayed until after delivery if the pregnancy has progressed further. It is important to avoid medications that can precipitate a crisis, such as metoclopramide or contrast media.
Thyroid disease
Normal changes in pregnancy
In pregnancy, the results of thyroid function tests are different from those in the non-pregnant population (Tables 1 and 2). Conditions that are associated with higher human chorionic gonadotropin (HCG) levels (including molar pregnancies, multiple pregnancies or hyperemesis gravidarum) can result in biochemical derangement that would otherwise suggest thyrotoxicosis.
Table 1.
Median trimester values for thyroid function tests.18
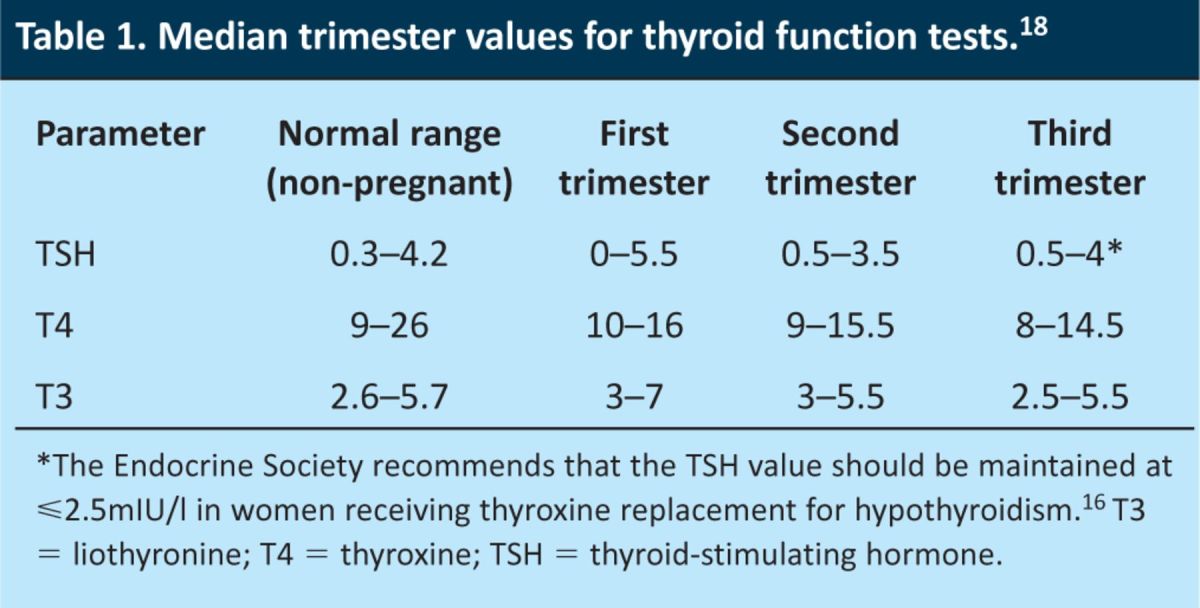
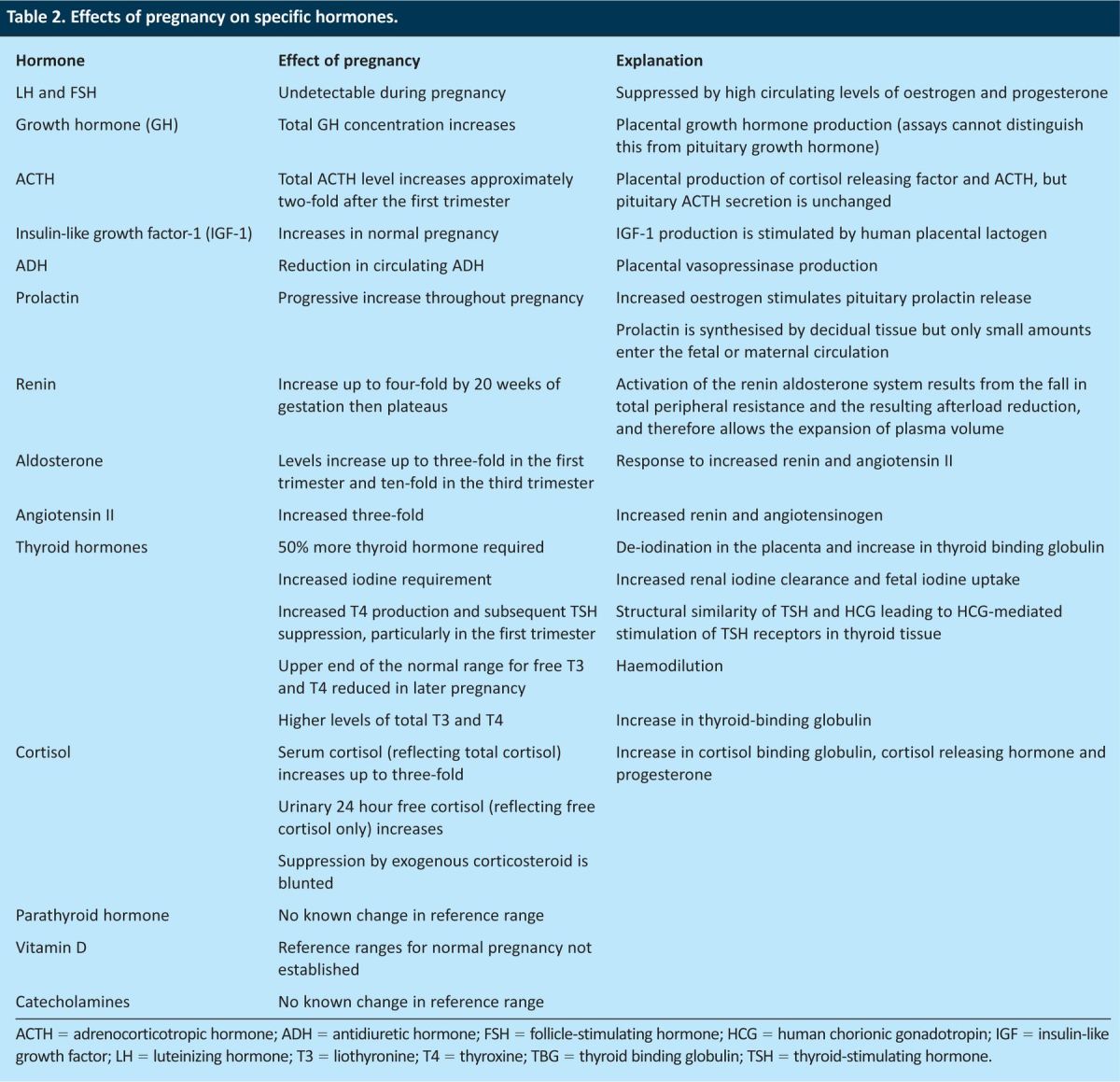
Table 2.
Effects of pregnancy on specific hormones.
Hypothyroidism
Hypothyroidism is present in up to 1% of pregnant women, with the majority having been identified and treated before conception. Many of the common clinical features of hypothyroidism occur in normal pregnancy, including lethargy, weight gain and constipation. More specific features include cold intolerance, bradycardia and delayed relaxation of tendon reflexes. Diagnosis is therefore based on raised thyroid-stimulating hormone (TSH) and low free thyroxine (fT4) concentrations.
It is not uncommon for women to require an increase in levothyroxine dose during pregnancy, which may reflect previous inadequate treatment. Empirical increases in dose are not however recommended in UK practice and dose adjustments should be guided by abnormal thyroid function tests.
Untreated hypothyroidism can lead to amenorrhea and infertility as a result of ovulation inhibition. After conception, it is associated with increased rates of miscarriage, anaemia and pre-eclampsia and with infants of low birth weight. Maternal iodine deficiency can have detrimental effects on the fetus if severe, and can result in neonatal hypothyroidism with abnormal cognitive and physical development. There are a number of studies that show cognitive changes in children of undertreated women, and lower birth weights in children of overtreated women,13–15 which therefore emphasise the importance of normalising thyroid function and avoiding over treatment. Subclinical hypothyroidism (TSH concentration >97.5th centile and normal T4) can be seen in 5% of the population and there is some evidence that this is associated with increased rates of preterm delivery and placental abruption.
Levothyroxine is safe and appropriate for use in pregnancy and can be started in patients diagnosed with hypothyroidism in pregnancy. It is advisable to change the treatment for women on other forms of thyroid replacement, such as liothyronine (T3), to T4 as T3 does not cross the placenta. This is important as the fetus is dependent on maternal thyroid hormone until approximately 12 weeks of gestation.
Thyroid function tests in hypothyroid women should be performed once in each trimester, and 4–6 weeks after any dose alteration. The Endocrine Society recommend aiming to maintain the TSH level at <2.5 mIU/l.16
Levothyroxine is excreted into breast milk, but not at levels sufficient to affect neonatal thyroid function and so breastfeeding can be undertaken as normal.
Hyperthyroidism
Hyperthyroidism complicates approximately 1 in 500 pregnancies. Pre-existing disease can lead to infertility if inadequately treated. If appropriate treatment is started with normalisation of thyroid function, then the occurrence of miscarriage, preterm labour and fetal growth restriction that are associated with uncontrolled disease are reduced. Autoimmune thyrotoxicosis might improve during pregnancy because of the occurrence of a degree of immunosuppression, but after delivery there is a risk of a flare.
Propylthiouracil (PTU) and carbimazole can be used in pregnancy. Both cross the placenta but neither is significantly teratogenic. Carbimazole is very rarely associated with aplasia cutis and choanal atresia, so PTU is the medication of choice for hyperthyroidism diagnosed in the first trimester. If a woman has been able to control her hyperthyroidism with carbimazole prior to pregnancy, however, she should remain on this drug so as to maintain good control. PTU can cause idiosyncratic hepatic reactions and both PTU and carbimazole can cause agranulocytosis. Both medications can be used by breastfeeding mothers, but carbimazole at high doses can result in neonatal thyroid dysfunction and therefore neonatal monitoring is required.
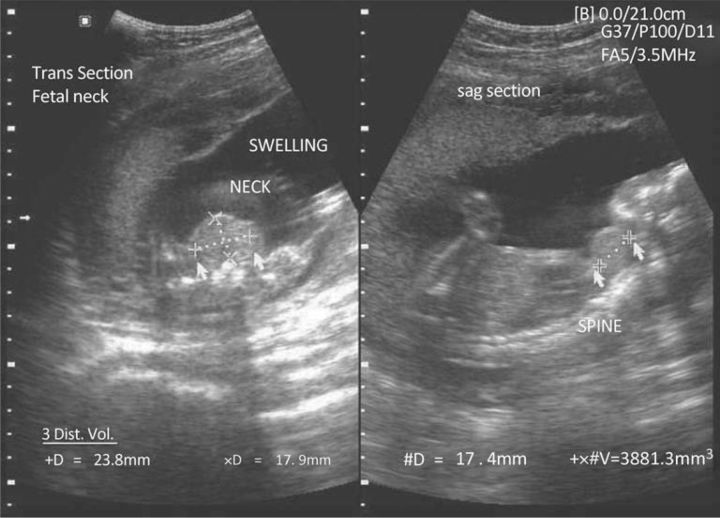
TSH receptor antibodies can cross the placenta and cause fetal hyperthyroidism in a small proportion of mothers who have Graves’ disease. This occurs more commonly in women who are not taking PTU or carbimazole, that is in those who have undergone radio-iodine treatment or surgery. This is because the anti-thyroid drugs cross the placenta and will protect the fetus from hyperthyroidism in women treated with PTU or carbimazole. Fetal ultrasound can be used to assess fetal tachycardia, hydrops, fetal goitre (Fig 1) and growth restriction. Untreated fetal hyperthyroidism as a result of transplacental passage of antibodies is associated with a mortality rate of up to 15%. If antibodies are detected, then cord and neonatal blood can be taken for assessment of thyroid function. It is important to screen the neonate for neonatal thyrotoxicosis. This typically presents soon after delivery and most commonly occurs in the babies of women taking PTU or carbimazole. The infant will continue to have maternal TSH-R antibodies for approximately three months but will no longer receive the drugs used to control hyperthyroidism via placental transfer.
Fig 1.
Fetal goitre identified on antenatal ultrasound.
Newly diagnosed hyperthyroidism in pregnancy requires prompt commencement of anti-thyroid medication. β-blockers can also be used if required. Surgery is rarely performed in pregnancy and radio-iodine is contraindicated as it can cause fetal hypothyroidism.
Calcium metabolism and parathyroid disease
Hyperparathyroidism
Primary hyperparathyroidism occurs in approximately eight women of child-bearing age in every 100,000. In pregnancy, hypertension or pre-eclampsia occur in up to 25% of cases.15 It can be complicated by maternal mortality resulting from pancreatitis or hypercalcaemic crises. There is a significant associated fetal morbidity with mortality rates of up to 40% when the maternal hypercalcaemia is severe (>3.5 mmol/l). There is also an increased risk of miscarriage, intrauterine growth restriction, stillbirth, neonatal tetany and neonatal death.
Hyperparathyroidism is diagnosed by identifying a high plasma-calcium concentration with a corresponding high or inappropriately normal parathyroid hormone concentration and high urinary calcium. Identification of hypercalcaemia in pregnancy can be difficult as the total calcium concentration can appear normal as a result of the lower albumin concentration. Isotope studies are contraindicated in pregnancy, but ultrasound can be used to identify adenomas.
Parathyroidectomy can be performed in pregnancy at most gestational ages. If surgery is not performed, management involves maintaining hydration and administering oral phosphates.
Conclusions
The investigation and management of endocrine conditions in pregnancy requires specialist and multidisciplinary input. Decisions about empirical treatment in the absence of a definitive diagnosis or on the timing and appropriateness of surgery during pregnancy can be challenging. The absence of large numbers of similar cases or trials of specific treatments in pregnancy mean that the evidence on which decisions are made is not as extensive as that relating to the non-pregnant population. It is therefore important to individualise treatment according to the specific needs of the particular patient and the condition involved.
References
- 1.Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf) 2010; 72: 377–82. 10.1111/j.1365-2265.2009.03667.x [DOI] [PubMed] [Google Scholar]
- 2.Overton CE, Davis CJ, West C, et al. High risk pregnancies in hypopituitary women. Hum Reprod 2002; 17: 1464–7. 10.1093/humrep/17.6.1464 [DOI] [PubMed] [Google Scholar]
- 3.Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK. Cushing's syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metab 2005; 90: 3077–83. [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004; 89: 1045–50. 10.1210/jc.2003-031337 [DOI] [PubMed] [Google Scholar]
- 5.Wilson M, Morganti AA, Zervoudakis I, et al. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am J Med 1980; 68: 97–104. 10.1016/0002-9343(80)90178-3 [DOI] [PubMed] [Google Scholar]
- 6.Suri D, Moran J, Hibbard JU, et al. Assessment of adrenal reserve in pregnancy: defining the normal response to the adrenocorticotropin stimulation test. J Clin Endocrinol Metab 2006; 91: 3866–72. 10.1210/jc.2006-1049 [DOI] [PubMed] [Google Scholar]
- 7.Hau J, Westergaard J, Teisner B, et al. Quantification of corticosteroid binding globulin by electroimmunoassay during human pregnancy. Arch Gynecol 1983; 223: 217–23. 10.1007/BF02114603 [DOI] [PubMed] [Google Scholar]
- 8.Feldman S, Billaud L, Thalabard JC, et al. Fertility in women with late-onset adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 1992; 74: 635–9. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Bahlberg H. What causes low rates of child-bearing in congenital adrenal hyperplasia? J Clin Endocrinol Metab 1999; 84: 1844–7. 10.1210/jc.84.6.1844 [DOI] [PubMed] [Google Scholar]
- 10.Falhammar H, Filipsson H, Holmdahl G, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2007; 92: 110–6. 10.1210/jc.2006-1350 [DOI] [PubMed] [Google Scholar]
- 11.New M, Carlson A, Obeid J, et al. Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 2001; 86: 5651–7. 10.1210/jc.86.12.5651 [DOI] [PubMed] [Google Scholar]
- 12.Lau P, Permezel M, Dawson P, et al. Phaeochromocytoma in pregnancy. Aust N Z J Obstet Gynaecol 1996; 36: 472–6. 10.1111/j.1479-828X.1996.tb02196.x [DOI] [PubMed] [Google Scholar]
- 13.Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol 1999; 50: 149–55. 10.1046/j.1365-2265.1999.00639.x [DOI] [PubMed] [Google Scholar]
- 14.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficieny during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341: 549–55. [DOI] [PubMed] [Google Scholar]
- 15.Blazer S. Maternal hypothyroidism may affect fetal growth and neonatal thyroid function. Obstet Gynecol 2003; 102: 232–41. 10.1016/S0029-7844(03)00513-1 [DOI] [PubMed] [Google Scholar]
- 16.De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 2543–65. 10.1210/jc.2011-2803 [DOI] [PubMed] [Google Scholar]
- 17.Schnatz PF, Thaxton S. Parathyroidectomy in the third trimester of pregnancy. Obstet Gynecol Surv 2005; 60: 672–82. 10.1097/01.ogx.0000180889.23678.fb [DOI] [PubMed] [Google Scholar]
- 18.Cotzias C, Wong SJ, Taylor E, et al. A study to establish gestation-specific reference intervals for thyroid function tests in normal singleton pregnancy. Eur J Obstet Gynecol Reprod Biol 2008; 137: 61–6. 10.1016/j.ejogrb.2007.10.007 [DOI] [PubMed] [Google Scholar]