Key points
Fresh frozen plasma (FFP) is perceived to reduce the risk of bleeding in coagulopathic patients, for whom it is commonly used
A prolonged prothrombin time (PT) or activated partial thromboplastin time (APTT) has a poor correlation with bleeding risk
When FFP is transfused, the improvement in clotting factors is minimal
There is no significant improvement in clinical outcome when FFP is transfused prophylactically
FFP is associated with risks, such as transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO), and, in light of the lack of evidence of a clear benefit in many circumstances, should be used with caution
Background
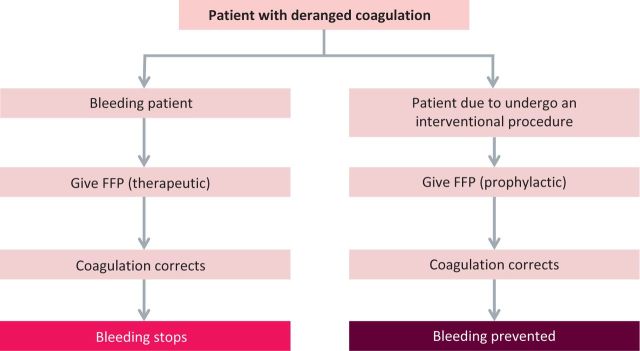
Fresh-frozen plasma (FFP) for transfusion contains a heterogeneous group of proteins, including procoagulant and inhibitory components of the coagulation system. FFP is either transfused prophylactically, which we discuss here, or therapeutically (which will be covered in a second article)1 for the treatment of patients who are bleeding (Fig 1).
Fig 1.
The main pathways and clinical rationale for fresh-frozen plasma (FFP) transfusion.
How useful are abnormalities of standard coagulation tests in predicting bleeding?
Decisions to transfuse FFP as prophylaxis are mainly in the context of abnormalities of standard coagulation tests, such as prothrombin time (PT) and activated partial thromboplastin time (APTT).2 However, PT and APTT results are dependent on reagent and laboratory quality controls and processes, and can be prolonged for several reasons not associated with a bleeding risk.3 For instance, APTT and PT depend on phospholipids, and the presence of antiphospholipid antibodies in the form of lupus anticoagulant can result in a prolonged APTT (and less commonly a prolonged PT). Another example is factor XII deficiency, which is associated with a prolonged APTT but conveys no additional bleeding risk.
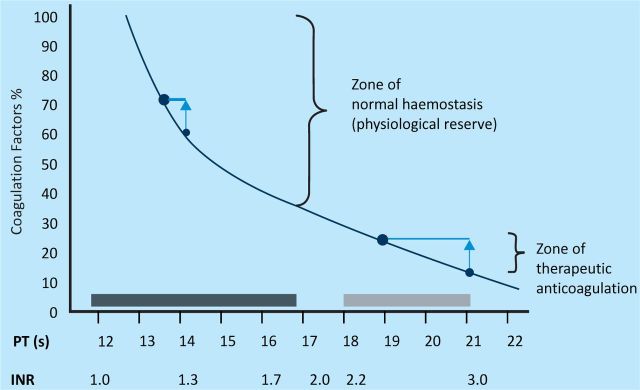
Another way of trying to appreciate the limitations of these standard tests is to consider the relation between deficiencies of factors (and bleeding risk) and PT (Fig 2).
Fig 2.
INR and coagulation reserve. There is no increase in bleeding risk until individual procoagulant factors fall below 30% of the expected level (dotted line). This corresponds to a prothrombin time (PT) of approximately 17 s. In the presence of multiple mild factor deficiencies, PT can be considerably more prolonged without any factor reaching this level. The black box represents the normal range for PT and the grey box represents the PT range for therapeutic anticoagulation. Fresh-frozen plasma (FFP) transfusion can result in mild improvements in factor levels. High levels of PT have a considerably greater effect (vertical arrows) than when PT is only mildly increased. Abbreviation: INR, international normalised ratio. Reproduced, with permission, from Callum and Dzik, 2010.4
A systematic review of whether a prolonged PT or APTT predicts bleeding after invasive procedures found one randomised controlled trial and 24 observational studies. There was no significant difference in the risk of bleeding between patients with a prolonged PT or APTT and those with normal clotting parameters in the setting of bronchoscopy, central vein cannulation, angiography or liver biopsy.5
Standard coagulation tests should not be interpreted in isolation, because the formation of a clot is dependent on several factors, including platelets, clotting factors, the patient's endothelium and rate of fibrinolysis.
Will FFP correct abnormalities in coagulation tests?
A related issue is the improvement in standard tests that one might see when FFP is transfused at conventional doses. Most patients who receive FFP have mild to moderate abnormalities of PT prolongation or international normalised ration (INR). In fact, all studies suggest that FFP does not significantly improve the results of coagulation tests. Median reduction in PT following FFP transfusion was −1.9 s (interquartile range −5.9–0.1; n=2701) in a recent UK study of plasma use.6 The Intensive Care Study of Coagulopathy (ISOC) was a prospective multicentre observational study of all sequentially admitted patients to UK general intensive care units. The study authors found a similar lack of overall changes. As expected, for the smaller number of cases where patients had higher baseline PT prolongation, greater reductions in standard coagulation tests were seen following the administration of FFP7 (Fig 2).
Will FFP improve clinical outcomes?
From the patient's perspective, the key outcome is whether use of FFP translates into improved clinical outcomes, not only the responses of standard coagulation tests. A systematic review of all randomised controlled trials evaluating the clinical use of FFP found no consistent clinical benefit across a range of clinical groups.8 Meta-analysis of trials in the cardiac section showed no significant difference between the experimental and control arms for the outcome of blood loss (–35.24 ml; 95% confidence interval −84.16–13.68 ml).
What are the risks of FFP transfusion?
Many risks of FFP transfusion are similar to those from transfusion of all blood components. However, there can be a higher incidence of transfusion-associated circulatory overload (TACO),9 transfusion-related acute lung injury (TRALI)10 and allergic reactions, including anaphylaxis, from FFP compared with other blood components. The risk of transmission of prion disease with plasma products is still uncertain, but is an important consideration for some countries, including the UK.11 Potential adverse events are of particular significance for patients receiving FFP for prophylaxis because they are exposed to the risks of FFP transfusion, even though it is questionable whether patients would be at greater risk of bleeding if plasma had not been transfused prophylactically.
What do these findings indicate for clinical practice?
The British Committee for Standards in Haematology (BCSH) guidelines for the use of FFP do not recommend the use of FFP for prophylaxis. However, despite this, FFP continues to be used in this setting.12 The national survey of FFP transfusion in England6 analysed 4,969 FFP transfusions given to patients in 190 hospitals, of which 93.3% were in adults and 6.7% in children or infants. In adult patients, 43% of all FFP transfusions were given in the absence of documented bleeding, as prophylaxis for abnormal coagulation tests or before procedures and/or surgery. Similarly, ISOC demonstrated that more than half of all FFP transfusions administered to patients who were critically ill were prophylactic.7 There are many reasons why clinicians continue to prescribe procoagulant agents, such as FFP, for prophylaxis despite a lack of evidence to support this approach. For instance, some clinicians might perceive that bleeding has been averted previously by the use of FFP and continue to use it for this purpose; some might feel a moral or psychological need to do everything possible to prevent bleeding, even in the absence of evidence.13
Conclusion
FFP continues to be widely used, despite a paucity of evidence of its efficacy. In particular, there is little evidence that it reduces the risk of bleeding in coagulopathic patients when used for prophylaxis. FFP transfusion is also associated with risks, such as TRALI and TACO. A more appropriate plasma transfusion strategy might be one that emphasises the therapeutic use in the face of bleeding (see reference 1), rather than prophylactic use in association with abnormalities of standard coagulation tests, which have limited predictive value for bleeding.
References
- 1.Desborough MJ, Stanworth SJ, Curry NS. Uses and abuses of fresh frozen plasma for the treatment of bleeding. Clin Med 2013; 13:xxx–xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzik WH. Predicting hemorrhage using preoperative coagulation screening assays. Curr Hematol Rep 2004; 3: 324–30. [PubMed] [Google Scholar]
- 3.Burns ER, Goldberg SN, Wenz B. Paradoxic effect of multiple mild coagulation factor deficiencies on the prothrombin time and activated partial thromboplastin time. Am J Clin Pathol 1993; 100: 94–8. [DOI] [PubMed] [Google Scholar]
- 4.Callum JL, Dzik WH. The use of blood components prior to invasive bedside procedures: a critical appraisal. In: Mintz PD. (ed), Transfusion therapy: clinical principles and practice, 3rd edn. Bethesda: AABB Press, 2010: 1–35. [Google Scholar]
- 5.Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005; 45: 1413–25. 10.1111/j.1537-2995.2005.00546.x [DOI] [PubMed] [Google Scholar]
- 6.Stanworth SJ, Grant-Casey J, Lowe D, et al. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion 2011; 51: 62–70. 10.1111/j.1537-2995.2010.02798.x [DOI] [PubMed] [Google Scholar]
- 7.Stanworth SJ, Walsh TS, Prescott RJ, et al. A national study of plasma use in critical care: clinical indications, dose and effect on prothrombin time. Crit Care 2011; 15: R108 10.1186/cc10129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Stanworth S, Hopewell S, et al. Is fresh frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 2012; 52: 1673–86. 10.1111/j.1537-2995.2011.03515.x [DOI] [PubMed] [Google Scholar]
- 9.Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion 2012; 52: 160–5. 10.1111/j.1537-2995.2011.03247.x [DOI] [PubMed] [Google Scholar]
- 10.Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2012; 119: 1757–67. 10.1182/blood-2011-08-370932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner ML, Ludlam CA. An update on the assessment and management of the risk of transmission of variant Creutzfeldt-Jakob disease by blood and plasma products. Br J Haematol 2009; 49: 14–23. 10.1111/j.1365-2141.2008.07376.x [DOI] [PubMed] [Google Scholar]
- 12.O'Shaughnessy DF, Atterbury C, Bolton-Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol 2004; 126: 11–28. [DOI] [PubMed] [Google Scholar]
- 13.Lipworth W, Kerridge I, Little M, et al. Evidence and desperation in off-label prescribing: recombinant factor VIIa. BMJ 2012; 344: d7926 10.1136/bmj.d7926 [DOI] [PubMed] [Google Scholar]