Key points
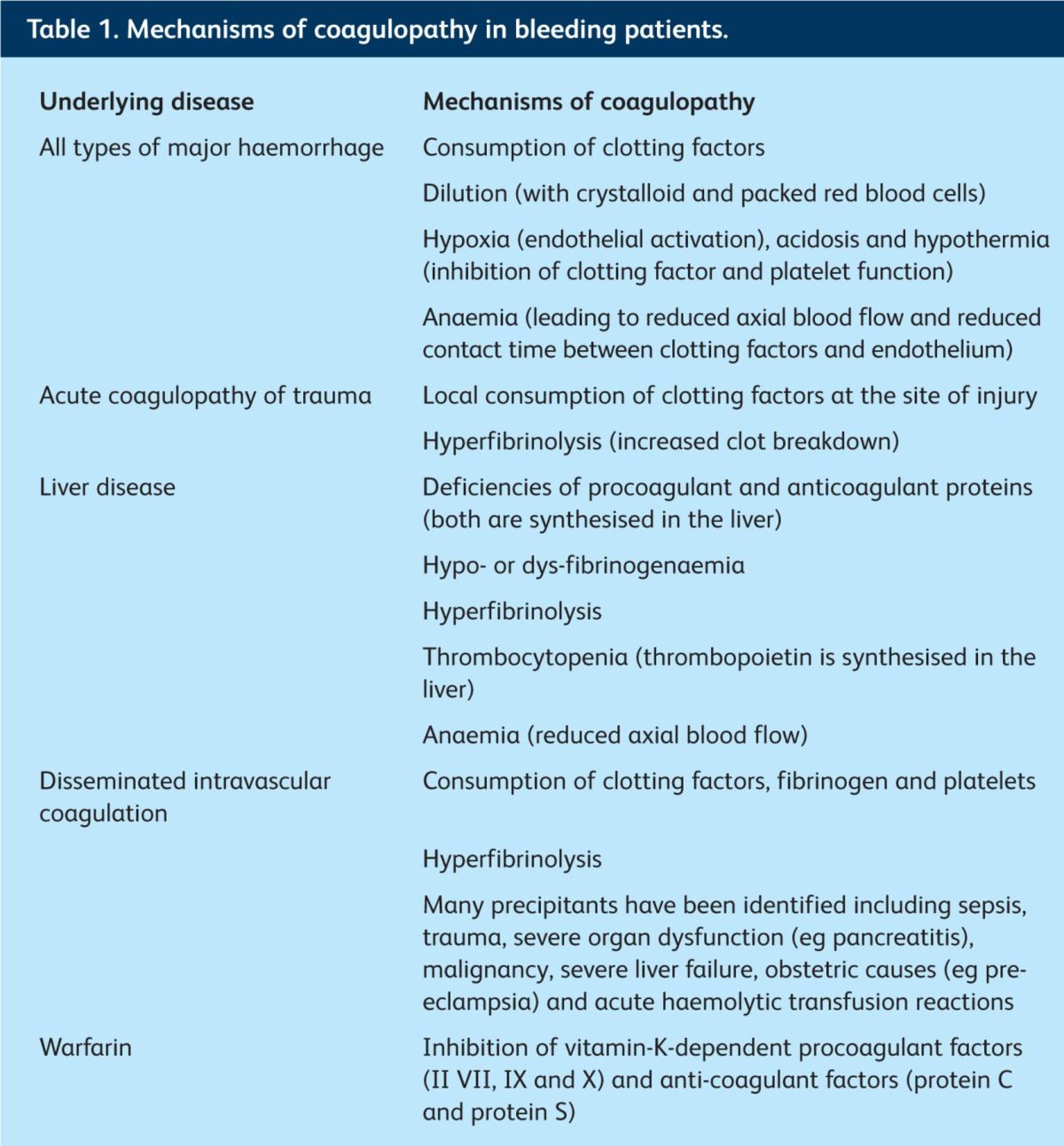
The causes of coagulopathy in patients with major bleeding are varied. They include the dilution and consumption of clotting factors, hyperfibrinolysis, hypoxia, acidosis and anaemia
Some of the protocols for treatment of coagulopathy in trauma advocate using a 1:1 ratio of fresh frozen plasma (FFP) and packed red blood cells (PRBC), but the evidence base for formula driven transfusion strategies is limited. There are concerns about extrapolating these strategies to patients with major bleeding outside trauma
Prothrombin complex concentrate (PCC) rather than FFP should be used to reverse warfarin over-anticoagulation
Patients with disseminated intravascular coagulation (DIC) should only be treated with FFP if they are bleeding
Background
Transfusion of packed red blood cells (PRBC) to maintain critical oxygen delivery can be lifesaving for patients who have major bleeding. In addition to PRBC, transfusion of blood components, including fresh frozen plasma (FFP), is frequently considered. There is, however, uncertainty about the optimal schedules for FFP administration or the best tests to assess effectiveness. Coagulopathy in patients with major bleeding (who have at least four units of PRBC transfused in the first 2–4 hours of bleeding)1 is typically defined and monitored by prothrombin time (PT) or international normalised ratio (INR), but these tests have recognised limitations in their ability to provide a relevant or global assessment of haemostatic potential.
The causes of coagulopathy in patients with major bleeding are varied and complex (Table 1). Historically, coagulopathy was thought to result from dilution of procoagulant clotting factors following infusion of PRBC and crystalloids; but it is now recognised that in trauma haemorrhage, a coagulopathy (the ‘acute coagulopathy of trauma’) might be evident before resuscitation fluids and PRBC have been administered.2 This discovery promotes early use of plasma for trauma patients, and explains why infusion of FFP before the PT becomes prolonged could be key to preventing (deteriorating) coagulopathy.
Table 1.
Mechanisms of coagulopathy in bleeding patients.
How does FFP treat coagulopathy?
Several mechanisms have been proposed for reduction in bleeding and improvement in coagulopathy seen in patients who are transfused with FFP.
FFP contains procoagulant and anti-fibrinolytic factors, which might replenish those lost through acute bleeding.
When patients are resuscitated with FFP rather than crystalloid or colloid, they are less likely to develop a dilutional coagulopathy.1
FFP contains fibrinogen, which replenishes losses during bleeding. It is possible that early fibrinogen use could be superior to FFP for patients with major bleeding, but this remains to be determined.3
Treating a coagulopathy is not the same as treating bleeding and there is no clear evidence that reversing coagulopathy results in a reduction in bleeding.4 There are well-recognised risks to FFP transfusion and one study found that when trauma patients who did not require a massive transfusion were transfused with FFP, there was a dose-related increase in adult respiratory distress syndrome, multi-organ failure, pneumonia and sepsis.5
Evidence to support FFP transfusion for bleeding patients
The majority of the evidence for the recent change in transfusion practice comes from studies of major bleeding in trauma patients. These studies often involve young patients who have no comorbidities, and their findings can be extrapolated only with caution to other patient groups who might have more complex medical needs – for example, to patients with liver disease or major gastrointestinal bleeding.
Major bleeding associated with trauma
In the past, ‘reactive’ infusions of FFP were typically prescribed only after abnormalities of PT were documented, but there is now a move towards the pro-active administration of FFP, aimed at preventing deterioration in coagulopathy. An empirical formula-driven approach is often used, defined by a high ratio of FFP transfusions to PRBC approaching 1:1. Although the results appear promising, the limited evidence to support improved patient outcomes and reduced mortality when this near 1:1 approach has been used should be recognised. The observational studies that have been carried out suffer from survivorship bias (only those that survived long enough were likely to have received FFP).6 When this was taken into account, Snyder et al7 found no significant reduction in mortality for patients who received high ratios of FFP. It is possible that concentrated sources of fibrinogen, such as cryoprecipitate, might prove superior to FFP in this setting, but their role remains to be determined.3
Major bleeding in settings outside trauma
At present, there is a tendency to apply trauma major haemorrhage protocols to other patient groups with bleeding. Therefore, FFP might commonly be given in combination with PRBC as part of a general massive-haemorrhage protocol. But many patients, such as those with gastrointestinal bleeding, are often older than the typical trauma patient and have very different comorbidities.
Patients with gastrointestinal bleeding might have underlying liver disease, which is characterised by complex changes in haemostasis (Table 1). A prolonged PT in a patient with liver disease is often assumed to represent ‘auto-anticoagulation’, but frequently this is not the case as many of these patients have a propensity towards thrombosis or have balanced haemostasis.8 Patients with liver disease often have other reasons to bleed, which can be more important than coagulopathy, such as increased portal venous pressure. If extrapolated from trauma practice, early or excessive use of plasma could lead to excessive volume replacement and exacerbate portal hypertension.
Disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) is predominantly a prothrombotic condition in which clotting factors, platelets and fibrinogen are rapidly consumed. The most important part of managing DIC is identifying and treating the underlying cause. A bleeding patient with DIC and a PT or activated partial thromboplastin time (APTT) greater than 1.5 times the upper limit of normal should be transfused with FFP, although there is no randomised controlled trial evidence to support this. FFP is not recommended for patients who are not bleeding.9 An exception to this rule is in the treatment of DIC in patients with acute promyelocytic leukaemia. These patients are at very high risk of bleeding and aggressive treatment of clotting abnormalities until PT or activated partial thromboplastin are normalised is indicated.10
Warfarin reversal
Major bleeding in a patient taking warfarin (defined as life- or limb-threatening bleeding that requires reversal in less than 6–8 hours) should, in preference, be treated with a combination of intravenous vitamin K and prothrombin complex concentrate (PCC).11 FFP is considerably less effective at reversing warfarin over-anticoagulation than PCC. A commonly referenced study of reversal of warfarin over-anticoagulation recruited and compared 12 patients who received vitamin K and FFP to 29 patients who received vitamin K and PCC. None of the patients who received FFP completely corrected their coagulopathy, whereas all the patients in the PCC group achieved this rapidly.12 There is, however, much less information on whether more rapid changes in laboratory-defined coagulopathy translate into improved clinical outcomes. FFP is only recommended for the reversal of warfarin over-anticoagulation if PCC is not readily available.
Conclusions
In summary, FFP is commonly used in patients with major haemorrhage. Outside trauma, there is little evidence to inform the optimal use of FFP, and there is a pressing need for new clinical studies to define best transfusion support. Locally agreed major haemorrhage protocols provide a structured framework to guide the transfusion of FFP and all blood components, ensuring that blood components can be accessed rapidly and appropriately. In an emergency situation, delivering blood components, including FFP, rapidly can present logistic difficulties, so the Care Quality Commission recommends that a major haemorrhage protocol should be in place in every hospital.13
References
- 1.Harris T, Thomas GO, Brohi K. Early fluid resuscitation in severe trauma. BMJ 2012; 345: e5752 10.1136/bmj.e5752 [DOI] [PubMed] [Google Scholar]
- 2.Hess JR. Blood and coagulation support in trauma care. Hematology Am Soc Hematol Educ Program 2007: 187–91. 10.1182/asheducation-2007.1.187 [DOI] [PubMed] [Google Scholar]
- 3.Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma haemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012; 10: 1042–51. 10.1111/j.1538-7836.2012.04752.x [DOI] [PubMed] [Google Scholar]
- 4.Curry N, Hopewell S, Doree C, et al. The acute management of trauma haemorrhage: a systematic review of randomized controlled trials. Crit Care 2011; 15: R92 10.1186/cc10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg 2010, 210: 957–65. 10.1016/j.jamcollsurg.2010.01.031 [DOI] [PubMed] [Google Scholar]
- 6.Curry NS, Davenport RA, Hunt BJ, et al. Transfusion strategies for traumatic coagulopathy. Blood Rev 2012; 26: 223–32. 10.1016/j.blre.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Snyder CW, Weinberg JA, McGwin G, Jr, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma 2011; 66: 358–62. 10.1097/TA.0b013e318196c3ac [DOI] [PubMed] [Google Scholar]
- 8.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011; 365: 147–56. [DOI] [PubMed] [Google Scholar]
- 9.Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 2009; 145: 24–33. [DOI] [PubMed] [Google Scholar]
- 10.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009; 113: 1875–91. 10.1182/blood-2008-04-150250 [DOI] [PubMed] [Google Scholar]
- 11.Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin — fourth edition. Br J Haematol 2011; 154: 311–24. [DOI] [PubMed] [Google Scholar]
- 12.Makris M, Greaves M, Phillips WS, et al. Emergency oral anticoagulation reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost 1997; 77: 477–80. [PubMed] [Google Scholar]
- 13.National Patient Safety Agency. Rapid Response Report. The transfusion of blood and blood components in an emergency. NPSA: London, 2010. www.nrls.npsa.nhs.uk/alerts/?entryid45=83659 [Accessed 26 February 2013]. [Google Scholar]


