Originally described by Laennac in 1819,1 bronchiectasis is a chronic, debilitating condition characterised by persistent cough, excessive sputum production and recurrent chest infections. The precise prevalence is unknown, but figures quoted vary from about 4 × 105 aged 18–34 years to 272 × 105 aged 75 years and over.2,3 Pathologically, there is abnormal permanent dilatation of the airways. This leads to impaired mucociliary clearance, which in turn leads to a vicious cycle of bacterial colonisation in normally sterile airways and excessive bronchial inflammation. This review explores current clinical practice for this complex condition.
Key Points.
Bronchiectasis should be considered in patients with a chronic, productive cough and a history of recurrent chest infections
The gold standard for diagnosis is computed tomography of the chest
The aetiology is unknown in up to 50% of cases and post-infective in up to 42%
The mainstays of treatment are regular chest physiotherapy, annual influenza vaccination and prompt administration of antibiotics for exacerbations
Long-term antibiotics should be considered for patients with recurrent chest infections impacting on their health-related quality of life
Diagnosis
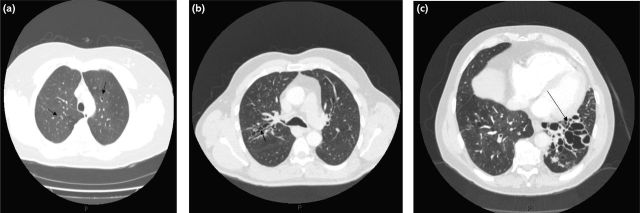
There is usually a history of a chronic productive cough and recurrent chest infections. There may be symptoms related to airways obstruction (wheeze and breathlessness), mucus plugging (chest pain) and also systemic symptoms.4 The diagnosis of bronchiectasis is confirmed radiologically with computed tomography of the chest. The defining characteristic is bronchial dilatation with the internal diameter of the bronchial lumen greater than that of the adjacent artery, categorised as tubular, varicose or cystic (Figs 1(a), 1(b) and 1(c)).5
Fig. 1.
(a) Tubular dilation of airways (see arrow); (b) varicose dilatation of airways (arrow shows irregular, dilated airway); (c) cystic dilatation of airways with thickened bronchi and mucus plugging (see arrow).
Aetiology
No underlying cause is identified in up to 50% of cases and is post-infective in up to 42%.6,7 The common causes, appropriate investigations and expected abnormal findings are listed in Table 1.6,7
Table 1.
Investigating the aetiology of non-cystic fibrosis (CF) bronchiectasis.
Assessment of severity
Clinical, radiological and microbiological features guide clinicians to the severity of bronchiectasis. These investigations not only provide clinicians with a quantitative assessment of disease severity but may also help in the management of both stable disease and exacerbations.
Sputum colour and volume. Colour is graded as mucoid, mucopurulent or purulent (Fig 2) and volume is measured over a 24-hour collection period. Patients with severe bronchiectasis usually have purulent sputum and volumes that may exceed 25 ml/day, even when stable.
Exacerbation frequency. In severe disease there are often multiple exacerbations (usually ≥3 a year) and inpatient management may be necessary.
Lung function. There can be advanced airflow obstruction in severe bronchiectasis, but there may also be restrictive or normal patterns.
Radiological findings. In severe disease bronchial dilatation is usually varicose or cystic with multiple lobes affected. There may be associated bronchial wall thickening, mucus plugging and subsegmental, segmental or lobar collapse.
Sputum microbiology. Most patients with severe bronchiectasis are chronically colonised with pathogenic organisms in their sputum when stable.8 Typical organisms include Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Moraxella catarrhalis and, in patients with advanced bronchiectasis, Pseudomonas aeruginosa. Patients colonised with P. aeruginosa have a poor health-related quality of life (HRQL), more severe airways obstruction and perhaps an accelerated decline in FEV1.9,10
Fig. 2.
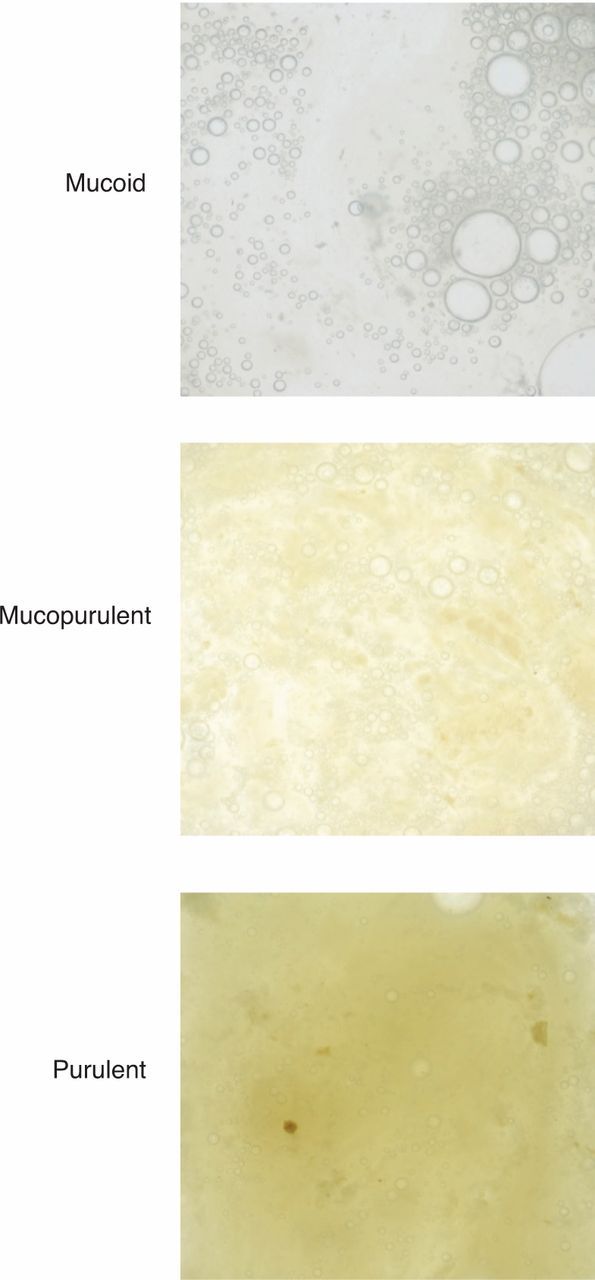
Sputum chart. Sputum is graded as mucoid, mucopurulent or purulent.
Management of stable disease
Management aims to reduce symptoms, limit exacerbations, preserve lung function and improve HRQL.
Patient education
Patients should be advised on smoking cessation, chest clearance techniques and long-term treatments. They should receive annual influenza vaccinations, the pneumococcal vaccination and prompt antibiotic treatment for infections.
Physiotherapy
The normal mucociliary clearance mechanism is impaired in bronchiectasis. Although randomised controlled trials (RCTs) assessing the efficacy of sputum clearance are lacking, physiotherapy is advised to promote clearance. Traditional postural drainage exercises can be difficult; newer techniques such as the active cycle breathing technique and assisted devices (eg the flutter) have been developed for patient ease. These have similar outcomes to postural drainage but are associated with greater patient preference.11,12 All patients should be reviewed by a specialist chest physiotherapist.
Adjuncts to physiotherapy. Several adjuncts have been proposed, including bronchodilator therapy, inhaled hyperosmolar agents (nebulised hypertonic saline, inhaled mannitol) and inhaled mucolytics (recombinant human DNase). Bronchodilator therapy (see below) may be used prior to chest physiotherapy to minimise bronchial hyperreactivity and improve airway clearance. Nebulised hypertonic 7% saline has been shown to yield greater sputum weights with greater ease and less viscosity, and small studies have shown that inhaled mannitol improves mucociliary clearance.13,14 Further studies are needed, but currently the latter two agents are not used in routine clinical practice.
Recombinant human DNase aims to reduce sputum viscosity, but a Cochrane review15 did not find enough evidence to support its regular use in bronchiectasis, and a multicentre study found it had a significant negative impact on FEV1.16
Bronchodilators
The role of bronchodilator therapy is yet to be established, but may be used as an adjunct to physiotherapy and to relieve breathlessness. If there is evidence of airways obstruction, reversibility testing should be performed to determine whether the patient could benefit from inhaled β2-agonists and/or anticholinergics. Both, however, may provide symptomatic relief of breathlessness, with or without an objective improvement in FEV1. A trial of the short-acting agents is recommended in the first instance in patients with impaired lung function and consideration of long-acting agents if clinical improvement.17,18
Inhaled corticosteroids
To date, RCTs of inhaled corticosteroids have shown a reduction in 24-hour sputum volume and improvement in HRQL, but no impact on FEV1 or exacerbation frequency.19–21 These studies used high-dose inhaled corticosteroids (fluticasone 500 μg bd or beclometasone 750 μg bd) but the optimal dose needs further clarification. A six-month trial of inhaled corticosteroids may be warranted, particularly for patients with evidence of airway obstruction and reversibility or with severe bronchiectasis.
Long-term antibiotics
The rationale for prescribing long-term antibiotics (oral or nebulised) is to reduce the bacterial burden in the airways, limiting inflammation and promoting healing of the bronchial tree.
Oral antibiotics. RCTs of long-term oral antibiotics for bronchiectasis are limited. In a 12-month Medical Research Council randomised placebo-controlled trial of tetracycline there was reduction in sputum volume, purulence and the number of days absent from work due to ill health.22 An eight-month randomised placebo-controlled trial of high-dose daily oral amoxicillin (3 g BD) found clinical improvement, reduction in 24-hour sputum volume, purulence and days absent from work, with the treatment well tolerated.23 Open label studies (≥6 months) assessing the role of macrolides have shown a reduction in exacerbation frequency.24,25 Oral treatment is inexpensive but systemic side effects are common.
Nebulised antibiotics. These offer a targeted therapy with limited systemic side effects. However, they are expensive and may be less well tolerated due to bronchospasm, even with adjunctive treatment with a β2-agonist. To date, the RCTs of long-term nebulised antibiotics have included only patients chronically colonised with P. aeruginosa. Two studies compared twice daily nebulised tobramycin with placebo, one cyclically (4 weeks treatment, 2 weeks off) and the other daily for six months.26,27 In both studies there was a reduction in bacterial density in the sputum but a small increased incidence of bronchospasm in the treatment arms. Another study compared nebulised ceftazidime and tobramycin with placebo for 12 months. Although there was no reduction in overall exacerbation frequency in the active group, the number and duration of hospital admissions for exacerbations were reduced.28
Further studies are needed to define who would benefit from long-term antibiotics and to determine the optimum treatment. Currently, long-term antibiotics may be indicated in those who have frequent exacerbations (usually ≥3/year) that impact on their HRQL.
Surgery
Indications for surgery include major haemoptysis or localised disease causing significant morbidity not responsive to medical management. Reported outcomes from surgery are good but studies were not randomised and in practice referral for surgical intervention is rare.29
Management of exacerbations
Prompt antibiotic treatment is recommended for patients presenting with increasing cough, sputum volume and purulence. There are many unanswered questions, including mode, choice and duration of antibiotic, monotherapy or dual agents and how best to assess response to treatment.
Antimicrobial agents
Sputum should be sent for microbiological culture at the start of all exacerbations and empirical treatment commenced immediately based on previous sputum microbiology, if available (see Table 2). Treatment should only be adjusted if there is no clinical response and should then be guided by the sputum culture and sensitivity results.
Table 2.
Recommended antibiotic therapy for exacerbations of bronchiectasis based on previous sputum microbiology.

Oral versus intravenous treatment
Oral antibiotic therapy should be used as first-line management unless:
the culture of pathogenic organisms sensitive only to intravenous agents
patients have clinical sepsis necessitating acute inpatient admission
there has been a failure of response to oral antimicrobials.
Monotherapy or dual antibiotic therapy?
Monotherapy is recommended for exacerbations due to S. pneumoniae, H. influenzae, M. catarrhalis, or methicillin-sensitive S. aureus (MSSA). To prevent further resistance emerging, two antibiotics are recommended for patients colonised with methicilin-resistant S. aureus (MRSA) and in patients with P. aeruginosa with multiple frequent exacerbations.
Duration
The optimum duration of treatment is unknown. In one study, inflammatory response returned to normal within a week of antimicrobial therapy, but symptomatic improvement has generally been seen in studies employing 10–14 days of treatment.30 At present, antibiotics are recommended for 10–14 days.
Other adjuncts to treatment
Regular chest physiotherapy is recommended. Patients with increased wheeze and dyspnoea may require optimisation of their bronchodilator therapy, including steroids.
Assessing response
It is helpful for clinicians to have endpoints to assess response to treatment. Bacterial clearance, 24-hour sputum volume, C-reactive protein and the St George's Respiratory Questionnaire (an HRQL questionnaire) are useful markers of treatment response, although the latter questionnaire is predominantly a research tool.31,32
Conclusions
There has been a resurgence of interest in the previously neglected condition of bronchiectasis. Ongoing and future RCTs will provide stronger evidence-based treatment for this chronic disabling disease.
Reference
- 1. Laennec RT. A treatise in the diseases of the chest and on mediate auscultation, 4th edn, 1819. Translation: Forbes J. London: Longman, 1834. 10.1097/00000441-183923470-00019 [DOI]
- 2. Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med 2005; 12:205–9. 10.1097/01.cpm.0000171422.98696.ed [DOI] [Google Scholar]
- 3. Twiss J, Metcalfe R, Edwards E, Byrnes C. New Zealand national incidence of bronchiectasis ‘too high’ for a developed country. Arch Dis Child 2005; 90:737–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006; 100:2183–9. 10.1016/j.rmed.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 5. Naidich DP, McCauley DI, Khouri NF, Stitik FP, Siegelman SS. Computed tomography of bronchiectasis. J Comput Assist Tomogr 1982; 6:437–44. 10.1097/00004728-198206000-00001 [DOI] [PubMed] [Google Scholar]
- 6. Nicotra MB, Rivera M, Dale AM. et al Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995; 108:955–61. 10.1378/chest.108.4.955 [DOI] [PubMed] [Google Scholar]
- 7. Pasteur MC, Helliwell SM, Houghton SJ. et al An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000;162(4 Pt 1)1277–84. [DOI] [PubMed] [Google Scholar]
- 8. Angrill J, Agusti C, de Celis R. et al Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002; 57:15–9. 10.1136/thorax.57.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies G, Wells AU, Doffman S, Watanabe S, Wilson R. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J 2006; 28:974–9. [DOI] [PubMed] [Google Scholar]
- 10. Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, Roman-Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132:1565–72. 10.1378/chest.07-0490 [DOI] [PubMed] [Google Scholar]
- 11. Patterson JE, Bradley JM, Hewitt O, Bradbury I, Elborn JS. Airway clearance in bronchiectasis: a randomized crossover trial of active cycle of breathing techniques versus Acapella. Respiration 2005; 72:239–42. 10.1159/000085363 [DOI] [PubMed] [Google Scholar]
- 12. Thompson CS, Harrison S, Ashley J, Day K, Smith DL. Randomised crossover study of the Flutter device and the active cycle of breathing technique in non-cystic fibrosis bronchiectasis. Thorax 2002; 57:446–8. 10.1136/thorax.57.5.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respir Med 2005; 99:27–31. [DOI] [PubMed] [Google Scholar]
- 14. Daviskas E, Anderson SD, Eberl S, Young IH. Effect of increasing doses of mannitol on mucus clearance in patients with bronchiectasis. Eur Respir J 2008; 31:765–72. 10.1183/09031936.00119707 [DOI] [PubMed] [Google Scholar]
- 15. Crockett AJ, Cranston JM, Latimer KM, Alpers JH. Mucolytics for bronchiectasis. Review. Cochrane Database Syst Rev 2001; (1):CD001289. [DOI] [PubMed]
- 16. O'Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998; 113:1329–34. [DOI] [PubMed] [Google Scholar]
- 17. Franco F, Sheikh A, Greenstone M. Short acting beta-2 agonists for bronchiectasis. Review. Cochrane Database Syst Rev 2003; (3):CD003572. [DOI] [PubMed]
- 18. Sheikh A, Nolan D, Greenstone M. Long-acting beta-2-agonists for bronchiectasis. Cochrane Database Syst Rev 2001;(4): CD002155. [DOI] [PMC free article] [PubMed]
- 19. Elborn JS, Johnston B, Allen F. et al Inhaled steroids in patients with bronchiectasis. Respir Med 1992; 86:121–4. 10.1016/S0954-6111(06)80227-1 [DOI] [PubMed] [Google Scholar]
- 20. Martinez-Garcia MA, Perpina-Tordera M, Roman-Sanchez P, Soler-Cataluna JJ. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med 2006; 100:1623–32. 10.1016/j.rmed.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 21. Tsang KW, Tan KC, Ho PL. et al Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax 2005;60:239–43 10.1136/thx.2002.003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prolonged antibiotic treatment of severe bronchiectasis; a report by a subcommittee of the Antibiotics Clinical Trials (non-tuberculous) Committee of the Medical Research Council. BMJ 1957; 2:255–9. 10.1136/bmj.2.5039.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Currie DC, Garbett ND, Chan KL. et al Double-blind randomized study of prolonged higher-dose oral amoxycillin in purulent bronchiectasis. Q J Med 1990; 76:799–816. [PubMed] [Google Scholar]
- 24. Cymbala AA, Edmonds LC, Bauer MA. et al The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treat Respir Med 2005; 4:117–22. 10.2165/00151829-200504020-00005 [DOI] [PubMed] [Google Scholar]
- 25. Davies G, Wilson R. Prophylactic antibiotic treatment of bronchiectasis with azithromycin. Thorax 2004; 59:540–1. [PMC free article] [PubMed] [Google Scholar]
- 26. Barker AF, Couch L, Fiel SB. et al Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med 2000;162(2 Pt 1)481–5. [DOI] [PubMed] [Google Scholar]
- 27. Drobnic ME, Sune P, Montoro JB, Ferrer A, Orriols R. Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa. Ann Pharmacother 2005; 39:39–44. [DOI] [PubMed] [Google Scholar]
- 28. Orriols R, Roig J, Ferrer J. et al Inhaled antibiotic therapy in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection by Pseudomonas aeruginosa. Respir Med 1999; 93:476–80. [DOI] [PubMed] [Google Scholar]
- 29. Balkanli K, Genc O, Dakak M. et al Surgical management of bronchiectasis: analysis and short-term results in 238 patients. Eur J Cardiothorac Surg 2003; 24:699–702. 10.1016/S1010-7940(03)00497-4 [DOI] [PubMed] [Google Scholar]
- 30. Tsang KW, Chan WM, Ho PL. et al A comparative study on the efficacy of levofloxacin and ceftazidime in acute exacerbation of bronchiectasis. Eur Respir J 1999; 14:1206–9. 10.1183/09031936.99.14512069 [DOI] [PubMed] [Google Scholar]
- 31. Murray MP, Turnbull K, Macquarrie S, Hill AT. Assessing response to treatment of exacerbations of bronchiectasis in adults. Eur Respir J 2009. Feb;33(2):312–8. 10.1183/09031936.00122508 [DOI] [PubMed] [Google Scholar]
- 32. Wilson CB, Jones PW, O'Leary CJ, Cole PJ, Wilson R. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997;156(2 Pt 1)536–41. [DOI] [PubMed] [Google Scholar]