The clinical and molecular investigation of familial neoplasia has provided important insight into the molecular mechanisms of neoplasia and enhanced the clinical management of affected individuals and their at-risk relatives across a broad range of human neoplasias. For most tumour types, only a small minority (typically up to 10%) of cases will occur in individuals with a high- or moderate-penetrance Mendelian disorder, but this proportion is much higher in patients with phaeochromocytoma and paraganglioma (PPGL). Thus, although the traditionally taught ‘10% rule’ suggests that only 10% of cases of PPGL are familial, it is now recognised that at least 25% of apparently sporadic cases of PPGL have a genetic basis.1,2
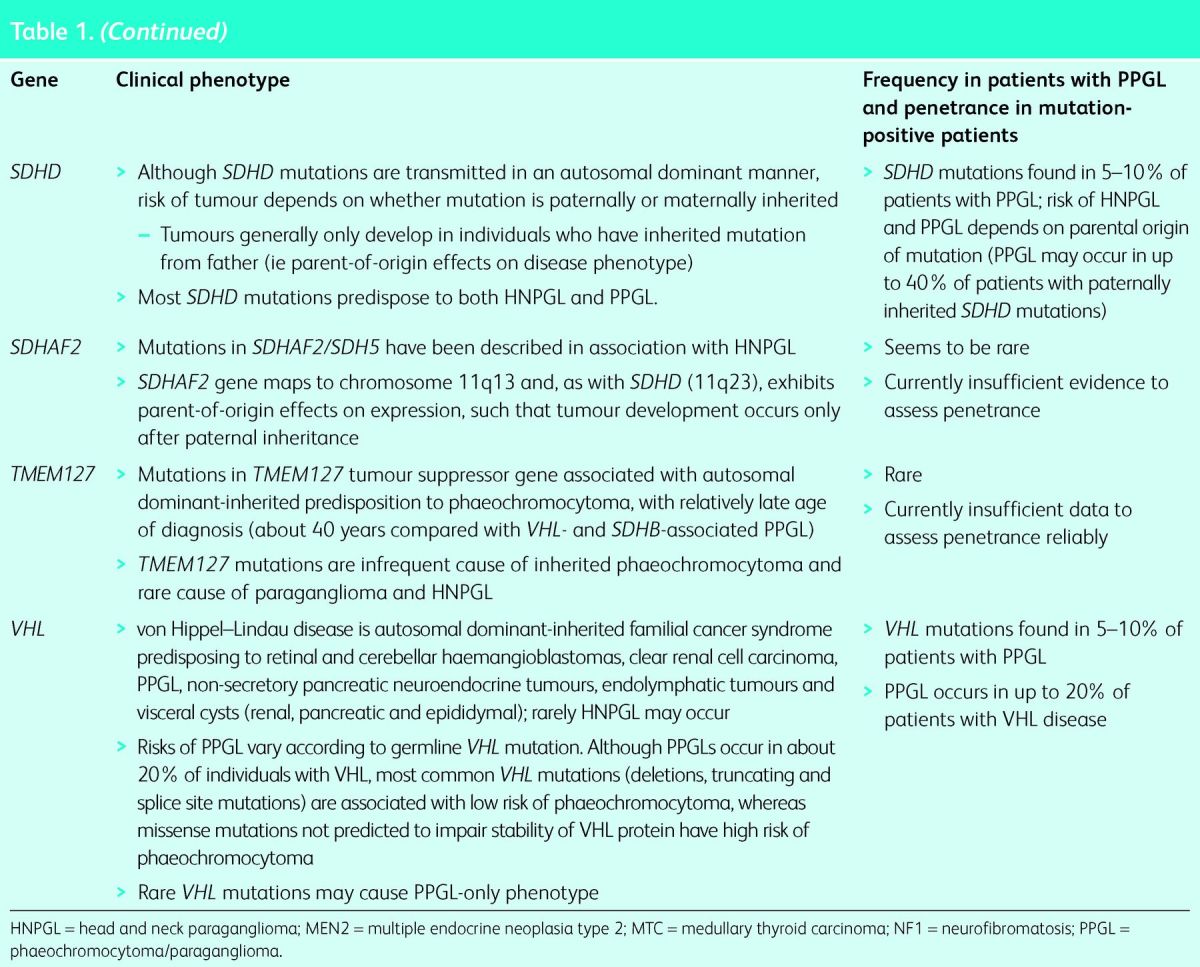
Currently, germline mutations in 12 genes are known to be associated with inherited PPGL, and it is likely that this number will increase. The genes and associated clinical features are summarised in Table 1, which highlights that the precise type of PPGL and the nature of other associated tumours vary according to the specific gene involved. The three longest recognised genetic causes of predisposition to PPGL are neurofibromatosis type 1 (NF1, von Recklinghausen's disease), multiple endocrine neoplasia 2 syndrome types A and B (MEN2A and MEN2B) and von Hippel–Lindau (VHL) disease.
Table 1.
Clinical characteristics associated with mutations in 12 genes associated with development of phaeochromocytoma–paraganglioma (PPGL) or head and neck paraganglioma (HNPGL) (see text for further details).

Patients with an underlying diagnosis of NF1 who present with phaeochromocytoma can usually be recognised clinically (mean age at diagnosis of phaeochromocytoma in NF1 is about 40 years, by which time the classic features of NF1 [café-au-lait spots, neurofibromas, axillary freckling, etc] are apparent). In MEN2A, phaeochromocytoma generally present in the context of a personal or family history of medullary thyroid carcinoma and in MEN2B patients will have other characteristic physical stigmata (eg mucosal neuromas, Marfanoid habitus).3,4 However, in VHL disease PPGL may be the only feature at presentation, so the threshold for molecular genetic analysis for VHL should reflect this (a subgroup of VHL gene mutations may predispose to PPGL but not other VHL-type tumours).4–6
Box 1. Case history illustrating pattern of inheritance seen with SDHD, SDHAF2 and MAX mutations.
Succinate dehydrogenase (SDH) is a heterotetrameric enzyme (subunits A, B, C and D) that is attached to the inner mitochondrial wall and has key roles in cellular energy production by virtue of its roles in the tricarboxylic acid (TCA, Krebs) cycle and as the complex II component of the electron transport chain. Mutations in genes that encode SDH subunits (SDHA, SDHB, SDHC or SDHD) or an associated protein (SDHAF2/SDH5) have been associated with inherited PPGL or head and neck paraganglioma (HNPGL),7–11 with most mutations occurring in SDHB and SDHD. Although germline mutations in SDHA, SDHB, SDHC, SDHD and SDHAF2 all compromise SDH activity, there are genotype–phenotype correlations. Mutations in SDHB cause PPGL more frequently than HNPGL, while the reverse is true for mutations in SDHD.12,13 Importantly, SDHB mutations are associated with an increased risk of malignant paraganglioma. Thus, although only about 10% of PPGLs are malignant, a germline SDHB mutation may be detected in up to 50% of individuals with a malignant paraganglioma.14,15 These genotype–phenotype correlations can be utilised to prioritise gene testing for germline mutations, although the increasing trend is to test multiple genes simultaneously rather than individual genes sequentially (see below).16–18 Rarer and more recently recognised genes implicated in inherited PPGL and HNPGL (SDHA, SDHC, SDHAF2, TMEM127, MAX, FH and HIF2A) are summarised in Table 1.10,11,19–21
To whom should genetic testing be offered?
Phaeochromocytoma is a rare tumour with an incidence of 2–8 cases per million per year. The personal and family history and clinical examination may reveal risk factors for inherited disease (see Table 2). The presence of two or more cases of PPGL or HNPGL in a family thus can usually be assumed to result from a familial mutation. In an individual with a phaeochromocytoma, paraganglioma or HNPGL, the presence of a family history of a tumour associated with a syndromic cause of PPGL should prompt genetic testing for the suspected syndrome. Familial PPGL, irrespective of the gene involved, is usually inherited as an autosomal dominant trait, so the children of a mutation carrier will have a 50% chance of having inherited the relevant PPGL gene mutation. However, for three genes (SDHD, SDHAF2 and MAX), the risk of a mutation carrier developing a tumour is dependent the parent from which the gene has been inherited – for these genes, clinical disease is generally seen only when the mutation has been inherited from the father.7,11,20 Hence, if a son were to inherit an SDHD mutation from his mother, his risk of developing a tumour is remote, but if any of his children were to inherit the SDHD mutation from him, they would be at significant risk of developing a tumour.
Table 2.
Features that suggest underlying genetic susceptibility in patients with phaeochromocytoma or paraganglioma (PPGL).

In addition to patients with syndromic causes of inherited PPGL recognisable by combinations of tumours as in VHL and MEN2A or by clinical phenotype as in NF1 and MEN2B, about 25% of individuals with apparently sporadic non-syndromic PPGL will harbour a clinically unsuspected disease gene mutation.1,2,22 In such cases, the lack of family history may result from a variety of causes: the mutation may have arisen de novo in the affected individual (up to 20% of cases of VHL disease) or may be non-penetrant in other relatives (recent work suggests that <50% of individuals with an SDHB mutation will develop a tumour); alternatively, it may be a reflection of parent-of-origin effects on penetrance (SDHD, SDHAF2 and MAX mutations). Among individuals with apparently sporadic non-syndromic PPGL or HNPGL, certain clinical features may point to those most likely to have a mutation. The presence of multiple tumours, extra-adrenal location, malignancy or early age at diagnosis thus all can indicate an increased likelihood of a germline mutation, and the presence or absence of such features has been used to stratify which individuals should be offered genetic testing.16,17 Although it had been recommended that all individuals with PPGL should be offered testing for SDHB, SDHD, VHL and RET mutations,2 such an approach is costly and most clinical centres have adopted a more focused approach to genetic testing based on clinical features. For example, testing is offered to all familial or syndromic cases and sporadic cases of PPGL with multiple tumours, malignancy, extra-adrenal location or age at onset <50 years.16,17 Evaluation of a UK-based genetic testing programme demonstrated that such ‘clinically targeted’ testing programmes can be cost efficient, but some, albeit infrequent, ‘low-risk’ individuals (eg those older than 50 years with isolated phaeochromocytoma) will harbour a germline mutation.22 Complete ascertainment of all mutation carriers thus requires universal testing (although the sensitivity of targeted testing programmes might be enhanced by the addition of tumour immunohistochemistry23).
How should genetic testing be performed?
Until recently, genetic testing for germline mutations in genes predisposing to PPGL and HNPGL involved sequentially testing single genes, prioritised according to clinical features, until a mutation was detected or until all genes (typically up to four – SDHB, SDHD, VHL and RET) tested negative. Such a testing protocol is expensive and might take longer than 6 months. Recently, advances in massive parallel sequencing technologies (next-generation sequencing/second-generation sequencing) have transformed the practice of DNA sequencing (initially in genome centres and research laboratories but more recently in diagnostic laboratories). Powerful but compact DNA sequencers thus allow simultaneous sequencing of multiple genes (‘gene panels’) in a single run at a much lower cost than conventional (Sanger) DNA-sequencing techniques. It has been estimated that a second-generation sequencing test for nine genes predisposing to phaeochromocytoma (MAX, RET, SDHA, SDHB, SDHC, SDHD, SDHAF2, TMEM127 and VHL) costs about £500 per sample, whereas sequencing of SDHB, SDHD, VHL and RET by conventional sequencing cost about £1,800.18 Although second-generation sequencing approaches have many advantages, there can be issues with sensitivity. Nevertheless, technological advances are widely expected to improve sensitivity and specificity, and sequencing costs will fall. Although it seems likely that further genes predisposing to PPGL and HNPGL remain to be identified, the cost of testing for these genes is anticipated to continue to fall and all or most patients with PPGL or HNPGL might be offered genetic testing in the medium term.
However, it is very important that all patients are counselled appropriately about the limitations of genetic testing. The analysis of genes predisposing to PPGL and HNPGL often yields the discovery of missense variants (‘variants of uncertain significance’), which cannot currently be interpreted reliably, thus producing an uninformative result. In addition, for patients with pathogenic mutations in newly recognised disease genes, information on long-term risks and optimum surveillance may be extremely limited, and this uncertainty can be difficult for patients and clinicians alike.
Conclusions
Both PPGL and HNPGL provide examples of how advances in molecular genetics can transform our knowledge of the genetic basis of a human disease. At least 12 genes are currently known to predispose to PPGL and HNPGL, with more likely to be found, but it is technologically and economically feasible that advances in DNA sequencing will enable universal testing of all individuals with PPGL or HNPGL. Such testing would have value for predicting the prognosis of individual patients (eg high risk of malignancy in those with an SDHB mutation or associated tumours in those with a VHL mutation). However, it is important that implementation of genetic testing does not outstrip our ability to interpret the results of genetic testing and our knowledge of the optimum strategies for managing mutation-positive individuals. It is important, therefore, that as genetic testing expands, there is a concerted effort to gather molecular and clinical data in order to provide a strong evidence base to guide future recommendations on the care of individuals susceptible to PPGL and HNPGL.
Key points.
About one-third of all patients with phaeochromocytoma and paraganglioma (PPGL) have an underlying genetic cause
Most patients with heritable PPGL do not have a positive family history
Mutations in at least 12 genes may cause inherited PPGL
Identification of individuals with germline mutations in inherited PPGL allows them (and their mutation-positive relatives) to be screened for development of PPGL and other relevant tumours
Clinical features (eg family history, multiple tumours, location, malignancy, young age at onset, etc) can be used to prioritise genetic testing, but complete ascertainment of inherited cases would require all cases to be tested
Advances in DNA sequencing technologies are enabling the development of more comprehensive genetic testing at lower cost
Acknowledgements
Phaeochromocytoma research in the Maher laboratory is sponsored by the British Heart Foundation. Apologies to the many authors whose work was unable to be cited because of lack of space.
References
- 1.Neumann HP, Bausch B, McWhinney SR, et al. Germ-line -mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002;346:1459–66. 10.1056/NEJMoa020152 [DOI] [PubMed] [Google Scholar]
- 2.Gimenez-Roqueplo AP, Lehnert H, Mannelli M, et al. Phaeochromocytoma, new genes and screening strategies. Clin Endocrinol 2006;65:699–705. 10.1111/j.1365-2265.2006.02714.x [DOI] [PubMed] [Google Scholar]
- 3.Bausch B, Koschker AC, Fassnacht M, et al. Comprehensive mutation scanning of NF1 in apparently sporadic cases of pheochromocytoma. J Clin Endocrinol Metab 2006;91:3478–81. 10.1210/jc.2006-0780 [DOI] [PubMed] [Google Scholar]
- 4.Guiral TS, Mulligan LM. Molecular implications of RET mutations for pheochromocytoma risk in multiple endocrine neoplasia 2. Ann NY Acad Sci 2006;1073:234–40. 10.1196/annals.1353.025 [DOI] [PubMed] [Google Scholar]
- 5.Woodward ER, Eng C, McMahon R, et al. Genetic predisposition to phaeochromocytoma: analysis of candidate genes GDNF, RET and VHL. Hum Mol Genet 1997;6:1051–6. 10.1093/hmg/6.7.1051 [DOI] [PubMed] [Google Scholar]
- 6.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet 2011;19:617–23. 10.1038/ejhg.2010.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000;287:848–51. 10.1126/science.287.5454.848 [DOI] [PubMed] [Google Scholar]
- 8.Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial -pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001;69:49–54. 10.1086/321282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiavi F, Boedeker CC, Bausch B, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA 2005;294:2057–63. 10.1001/jama.294.16.2057 [DOI] [PubMed] [Google Scholar]
- 10.Burnichon N, Brière JJ, Libé R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 2010;19:3011–20. 10.1093/hmg/ddq206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 2009;325:1139–42. 10.1126/science.1175689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical -features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004;292:943–51. 10.1001/jama.292.8.943 [DOI] [PubMed] [Google Scholar]
- 13.Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and -genotype-phenotype-proteotype analysis in 358 patients with germ-line mutations in SDHB and SDHD. Hum Mutat 2010;31:41–51. 10.1002/humu.21136 [DOI] [PubMed] [Google Scholar]
- 14.Gimenez-Roqueplo AP, Favier J, Rustin P, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res 2003;63:5615–21. [PubMed] [Google Scholar]
- 15.Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine–producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab 2006;91:4505–9. 10.1210/jc.2006-0423 [DOI] [PubMed] [Google Scholar]
- 16.Erlic Z, Rybicki L, Peczkowska M, et al. Clinical predictors and -algorithm for the genetic diagnosis of phaeochromocytoma patients. Clin Cancer Res 2009;15:6389–95. 10.1158/1078-0432.CCR-09-1237 [DOI] [PubMed] [Google Scholar]
- 17.Jafri M, Maher ER. The genetics of phaeochromocytoma: using clinical features to guide genetic testing. Eur J Endocrinol 2012;166:151–8. 10.1530/EJE-11-0497 [DOI] [PubMed] [Google Scholar]
- 18.Rattenberry E, Vialard L, Yeung A, et al. A comprehensive next -generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2013;98:E1248–56. 10.1210/jc.2013-1319 [DOI] [PubMed] [Google Scholar]
- 19.Yao L, Schiavi F, Cascon A, et al. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA 2010;304:2611–9. 10.1001/jama.2010.1830 [DOI] [PubMed] [Google Scholar]
- 20.Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 2011;43:663–7. 10.1038/ng.861 [DOI] [PubMed] [Google Scholar]
- 21.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 2012;367:922–30. 10.1056/NEJMoa1205119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafri M, Whitworth J, Rattenberry E, et al. Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin Endocrinol 2013;78:898–906. 10.1111/cen.12074 [DOI] [PubMed] [Google Scholar]
- 23.van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: retrospective and prospective analysis. Lancet Oncol 2009;10:764–71. 10.1016/S1470-2045(09)70164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]



