Abstract
Although motor fluctuation can often be severe in Parkinson's disease (PD), it is rare for an ‘off period’ to result in coma. The case presented here is of a patient with longstanding PD who was admitted to our hospital with a Glasgow Coma Scale of three after missing just one or two doses of her medication. Investigation for infective, neurovascular and metabolic causes of coma were negative and the patient recovered very rapidly following reinstitution of dopaminergic therapy via nasogastric tube. This case highlights how florid the presentation of motor fluctuations in PD can be and the importance of restarting treatment as quickly as possible. Guidance is provided on how to administer dopaminergic medications in patients who are unable to swallow.
KEYWORDS : Parkinson’s disease, coma, wearing off, acute akinesia, high protein diet
Case presentation
A 67-year-old retired doctor, Dr N, with a 23-year history of Parkinson's disease (PD) and type 2 diabetes, presented to the emergency department (ED) on New Year's Day. She had hosted a large New Year's Eve party the preceding evening and recalled taking her medication at 10pm on New Year's Eve, although it was unclear whether she had taken her tablets at 11am that morning. Since diagnosis, she had led a reasonably independent life on dopaminergic medication, although she no longer drove a car. Shortly prior to admission, Dr N had experienced an increased frequency of falls. She modulated her own treatment according to symptoms: Sinemet Plus (co-careldopa) 25/100 mg four times daily, pramipexole modified release 0.7 mg three times daily (TDS) and amantadine 100 mg TDS, in addition to omeprazole and metformin. She had consumed less fluid than usual over the New Year period, causing dehydration and constipation. On New Year's Day she had gone to sleep at 11am after cooking lunch. At 2pm her son (also a doctor) could not rouse her and called an ambulance. Paramedics excluded hypoglycaemia (blood sugar 9.0 mmol/l) and arranged transfer to hospital. In the ED at 5pm, Dr N had a Glasgow Coma Scale (GCS) score of 5/15 (E1 M3 V1). Her blood pressure was 130/78 mmHg, pulse 84 beats per minute and blood oxygen saturation 98% on room air. There was no focal neurological deficit. A computerised tomographic (CT) head scan revealed a mild degree of cerebral atrophy, but was otherwise normal. Routine haematology and biochemistry investigations were normal. After exclusion of an intracranial haemorrhage, she was referred to the medical team.
What is the differential diagnosis and the most likely diagnosis?
A subdural haemorrhage was ruled out by a normal CT head scan. While acute ischaemic stroke was a possibility in view of the patient's age and the presence of type 2 diabetes, the absence of focal neurology made this unlikely. Normal peripheral white cell count and C-reactive protein made bacterial meningitis unlikely; intravenous aciclovir was commenced to cover possible viral encephalitis. The suspicion of missed medication, possible malabsorption (due to constipation and high protein diet) as well as the presence of rigidity made a severe ‘wearing off’ phenomenon the most likely diagnosis.
What is the initial management?
The intensive care unit (ICU) team assessed the patient's airway, which was patent, and noted that she was haemodynamically stable. The neurology team advised nasogastric (NG) tube insertion in order that her usual anti-Parkinsonian medication could be administered. Further review at 10.40pm revealed her GCS score to be 3/15; the patient was more rigid, but still without focal neurological signs; specifically, her reflexes were diminished and her plantars were downgoing. Further review by the ICU team indicated that, as the airway was patent, the patient could be managed on the medical assessment unit.
Dispersible madopar (co-beneldopa) was administered via nasogastric tube at approximately 3am on 2 January; there was a delay as the first NG tube position, as seen on CXR was not in the correct position, hence CXR was repeated after readjustment. There was a plan to perform a lumbar puncture if the GCS score did not improve substantially on dopamine replacement.
Case progression
By 6am on 2 January, the patient's GCS score had improved to 6/15 (E1 V1 M4), and at 8.30am she was following commands and answering questions, although still drowsy. By 10am, the patient's GCS score had improved to 15/15; the NG tube was removed later on that day. Aciclovir was discontinued.
Discussion
Parkinson's disease (PD) is a progressive neurodegenerative condition for which the mainstay of therapy is dopamine replacement in the form of levodopa.1 Dr N was on levodopa therapy (Sinemet Plus) as well as two dopamine agonists (pramipexole and amantadine). ‘Wearing off’ is a phenomenon where there is a decline in the duration of benefit from each dose and is seen in the majority of patients; it can occur after 5–6 months of treatment.1 Toru et al described a case of severe motor dysfunction, with extreme rigidity and impairment of volitional movement to the point where the patient was unable to speak, open their eyes or react to external stimuli following withdrawal of dopaminergic medication.2 However, this acute akinesia with transient unresponsiveness can occur despite continued treatment. Triggers include change in drug regime, surgery, intercurrent infection or gastrointestinal disease.3
Dr N was thought to have taken no dopaminergic medication in more than 16 hours. Fatigue and a large protein-rich Chinese meal consumed shortly before her last confirmed dose of treatment may have contributed to the decline in her condition. High protein content in the diet is thought to antagonise absorption and bioavailability of oral dopaminergic treatment.4
Severe motor ‘wearing off’ phenomena can be life threatening, in extreme cases resembling neuroleptic malignant syndrome with confusion, hyperthermia and rhabdomyolysis.3 Patients with severe ‘wearing off’ phenomena may not be able to take oral dopamine replacement therapy and so other routes of administration may be required.
Many NHS trusts have guidelines for the administration of anti-Parkinsonian medication in patients who are unable to swallow. The majority recommend conversion of levodopa to dispersible Madopar, administered via NG tube; with controlled release (CR) preparations, the dose of levodopa is decreased by 30%, as CR preparations have lower bioavailability.5 Conversely, for patients taking catechol-O-methyltransferase (COMT) inhibitors, the dose is increased by 30%.5
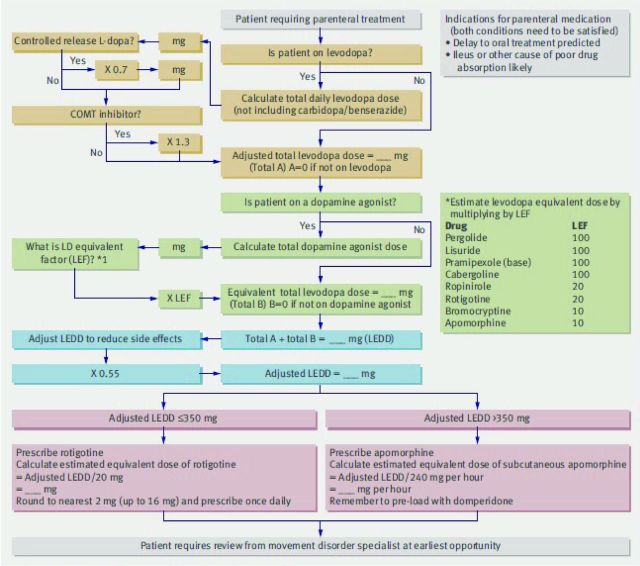
Apomorphine (subcutaneous) and rotigotine (transdermal) are dopamine agonists that are used when the oral route is not viable. Dopamine agonists can be converted to transdermal rotigotine using an algorithm published by Brennan and Genever (Fig 1).5 Where NG and transdermal treatments are both ineffective, subcutaneous apomorphine can be used, but caution is required in patients with dysphagia as this is emetogenic.
Fig 1.
Algorithm for estimating parenteral doses of drugs for Parkinson's disease. Reproduced with permission from Brennan et al (2010).5 COMT = catechol-O-methyltransferase; L-dopa = levodopa; LEDD = levodopa equivalent daily dose; LEF = levodopa equivalent factor.
While decreased consciousness secondary to wearing off phenomenon is often the most likely diagnosis, there are other serious differential diagnoses to be considered. Focused neurological examination is essential; urgent brain imaging and lumbar puncture are often indicated. Empirical antimicrobials and supportive therapy may also be required while awaiting results or response to dopaminergic medication. Early involvement of the ICU team for potential airway support is advised, as is liaison with neurology services, particularly clinical nurse specialists where available, and pharmacists for advice regarding suitable preparation of dopamine replacement therapy.
Key points.
Patients with Parkinson's disease (PD) can present with a ‘wearing off’ phenomenon that can mimic coma and be life threatening
Triggers include change in drug regime, infection, gastrointestinal disease, surgery and fatigue
A protein-rich diet can affect absorption of anti-Parkinsonian medication
Wearing off is treated with dopamine therapy via a nasogastric tube; transdermal rotigotine or apomorphine infusions can also be used
Clinicians need to be aware of other serious, life-threatening diagnoses in patients with PD who present with coma, eg meningitis, encephalitis, stroke
Acknowledgements
The authors are grateful to Dr Richard Genever for allowing us to use his algorithm for estimating parenteral doses of drugs in Parkinson's disease.
References
- 1.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–508. 10.1056/NEJMoa033447 [DOI] [PubMed] [Google Scholar]
- 2.Toru M, Matsuda O, Makiguchi K, Sugano K. Neuroleptic malignant syndrome-like state following a withdrawal of antiparkinsonian drugs. J Nerv Ment Dis 1981;169:324–7. 10.1097/00005053-198105000-00011 [DOI] [PubMed] [Google Scholar]
- 3.Onofrj M, Thomas A. Acute akinesia in Parkinson disease. Neurology 2005;64:1162–9. 10.1212/01.WNL.0000157058.17871.7B [DOI] [PubMed] [Google Scholar]
- 4.Pincus JH, Barry KM. Dietary method for reducing fluctuations in Parkinson's disease. Yale J Biol Med 1987;60:133–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan KA, Genever RW. Managing Parkinson's disease during -surgery. BMJ 2010;341:c5718. 10.1136/bmj.c5718 [DOI] [PubMed] [Google Scholar]