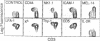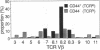Abstract
In the present report we describe a CD4+8- heat stable antigen-negative (HSA-) thymocyte subpopulation that expresses a distinguishably low density of alpha beta T-cell antigen receptors (TCRlo) from the majority of CD4+8- high-density TCR (TCRhi) mature-type thymocytes. This subpopulation appears relatively late in life. Analysis of MEL-14, Pgp-1 (CD44), ICAM-1 (CD54), and NK1.1 expression on this subpopulation revealed that the CD4+8- TCRlo population was a population having unique characteristics (MEL-14-, CD44+, ICAM-1+, and NK1.1+) compared to the CD4+8- TCRhi thymocytes, most of which are MEL-14+, CD44-, ICAM-1-, and NK1.1-. When TCR beta-chain variable region (V beta) usage was analyzed, this thymic population expressed predominantly products of V beta 7 and V beta 8.2 TCR gene families. Interestingly, cells with V beta 8.1 TCRs, which are reactive to Mls-1a antigens, were not eliminated from the CD4+8- HSA- TCRlo subpopulation but had been eliminated from the major CD4+8- HSA- TCRhi subpopulation in Mls-1a strains. A subset with a phenotype similar to the CD4+8- HSA- TCRlo thymocytes was also identified primarily in bone marrow, and this subset constituted approximately half of the CD4+ T cells in the bone marrow. The CD4+8- HSA- TCRlo cells showed extremely high proliferative responses to immobilized anti-TCR antibody but generated negligible responses to allogeneic H-2 antigens compared to the responses generated by the major CD4+8- HSA- CD3hi cells. However, the CD4+8- HSA- TCRlo cells in Mls-1b mice mounted vigorous proliferative responses to Mls-1a antigens but not in Mls-1a mice. The properties of this T-cell subset suggest that these cells belong to a lineage distinct from the major T-cell population.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus D. B., Surh C. D., Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991 May 1;173(5):1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Fukushi N., Hatakeyama S., Ogasawara K., Iwabuchi K., Iwabuchi C., Negishi I., Good R. A., Onoé K. Sequential analysis of the thymocyte differentiation in fully allogeneic bone marrow chimera in mice. II. Further characterization of the CD4+ or CD8+ single positive thymocytes. Immunobiology. 1990 Feb;180(2-3):167–183. doi: 10.1016/S0171-2985(11)80326-8. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Schwartz R. H. CD4+ and CD8+ T cells acquire specific lymphokine secretion potentials during thymic maturation. Nature. 1991 Sep 5;353(6339):68–71. doi: 10.1038/353068a0. [DOI] [PubMed] [Google Scholar]
- Bill J., Kanagawa O., Linten J., Utsunomiya Y., Palmer E. Class I and class II MHC gene products differentially affect the fate of V beta 5 bearing thymocytes. J Mol Cell Immunol. 1990;4(5):269–280. [PubMed] [Google Scholar]
- Bill J., Kanagawa O., Woodland D. L., Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Phenotypic identification of memory cytolytic T lymphocytes in a subset of Lyt-2+ cells. J Immunol. 1987 Feb 15;138(4):1009–1013. [PubMed] [Google Scholar]
- Butturini A., Gale R. P. T cell depletion in bone marrow transplantation for leukemia: current results and future directions. Bone Marrow Transplant. 1988 May;3(3):185–192. [PubMed] [Google Scholar]
- Fukushi N., Arase H., Wang B., Ogasawara K., Gotohda T., Good R. A., Onoé K. Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6301–6305. doi: 10.1073/pnas.87.16.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Hackett J., Jr, Tutt M., Lipscomb M., Bennett M., Koo G., Kumar V. Origin and differentiation of natural killer cells. II. Functional and morphologic studies of purified NK-1.1+ cells. J Immunol. 1986 Apr 15;136(8):3124–3131. [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T. M., Gallatin W. M., Weissman I. L., Dailey M. O. Down-regulation of homing receptors after T cell activation. J Immunol. 1988 Dec 15;141(12):4110–4117. [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada C. Y., Holzmann B., Guidos C., Palmer E., Weissman I. L. Characterization of a rat monoclonal antibody specific for a determinant encoded by the V beta 7 gene segment. Depletion of V beta 7+ T cells in mice with Mls-1a haplotype. J Immunol. 1990 May 1;144(9):3473–3477. [PubMed] [Google Scholar]
- Onoé K., Fernandes G., Good R. A. Humoral and cell-mediated immune responses in fully allogeneic bone marrow chimera in mice. J Exp Med. 1980 Jan 1;151(1):115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Prieto J., Takei F., Gendelman R., Christenson B., Biberfeld P., Patarroyo M. MALA-2, mouse homologue of human adhesion molecule ICAM-1 (CD54). Eur J Immunol. 1989 Sep;19(9):1551–1557. doi: 10.1002/eji.1830190906. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Jenkins M., Dinh Q., Fowlkes B. J. The majority of CD4+8- thymocytes are functionally immature. J Immunol. 1991 Sep 15;147(6):1779–1785. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Takahama Y., Sharrow S. O., Singer A. Expression of an unusual T cell receptor (TCR)-V beta repertoire by Ly-6C+ subpopulations of CD4+ and/or CD8+ thymocytes. Evidence for a developmental relationship between Ly-6C+ thymocytes and CD4-CD8-TCR-alpha beta+ thymocytes. J Immunol. 1991 Nov 1;147(9):2883–2891. [PubMed] [Google Scholar]
- Tomonari K., Hederer R., Hengartner H. Positive selection of Tcrb-V10b+ T cells. Immunogenetics. 1992;35(1):9–15. doi: 10.1007/BF00216621. [DOI] [PubMed] [Google Scholar]
- Tomonari K., Lovering E., Spencer S. Correlation between the V beta 4+ CD8+ T-cell population and the H-2d haplotype. Immunogenetics. 1990;31(5-6):333–339. doi: 10.1007/BF02115007. [DOI] [PubMed] [Google Scholar]
- Truitt R. L., Shih C. Y., Lefever A. V., Tempelis L. D., Andreani M., Bortin M. M. Characterization of alloimmunization-induced T lymphocytes reactive against AKR leukemia in vitro and correlation with graft-vs-leukemia activity in vivo. J Immunol. 1983 Oct;131(4):2050–2058. [PubMed] [Google Scholar]
- Tutt M. M., Kuziel W. A., Hackett J., Jr, Bennett M., Tucker P. W., Kumar V. Murine natural killer cells do not express functional transcripts of the alpha-, beta-, or gamma-chain genes of the T cell receptor. J Immunol. 1986 Nov 1;137(9):2998–3001. [PubMed] [Google Scholar]
- Utsunomiya Y., Kosaka H., Kanagawa O. Differential reactivity of V beta 9 T cells to minor lymphocyte stimulating antigen in vitro and in vivo. Eur J Immunol. 1991 Apr;21(4):1007–1011. doi: 10.1002/eji.1830210422. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Hoffman P. A., Gallatin W. M. Expression of lymphocyte adhesion receptors for high endothelium in primates. Anatomic partitioning and linkage to activation. J Immunol. 1989 May 15;142(10):3416–3422. [PubMed] [Google Scholar]
- Wilson A., Day L. M., Scollay R., Shortman K. Subpopulations of mature murine thymocytes: properties of CD4-CD8+ and CD4+CD8- thymocytes lacking the heat-stable antigen. Cell Immunol. 1988 Dec;117(2):312–326. doi: 10.1016/0008-8749(88)90121-9. [DOI] [PubMed] [Google Scholar]