Abstract
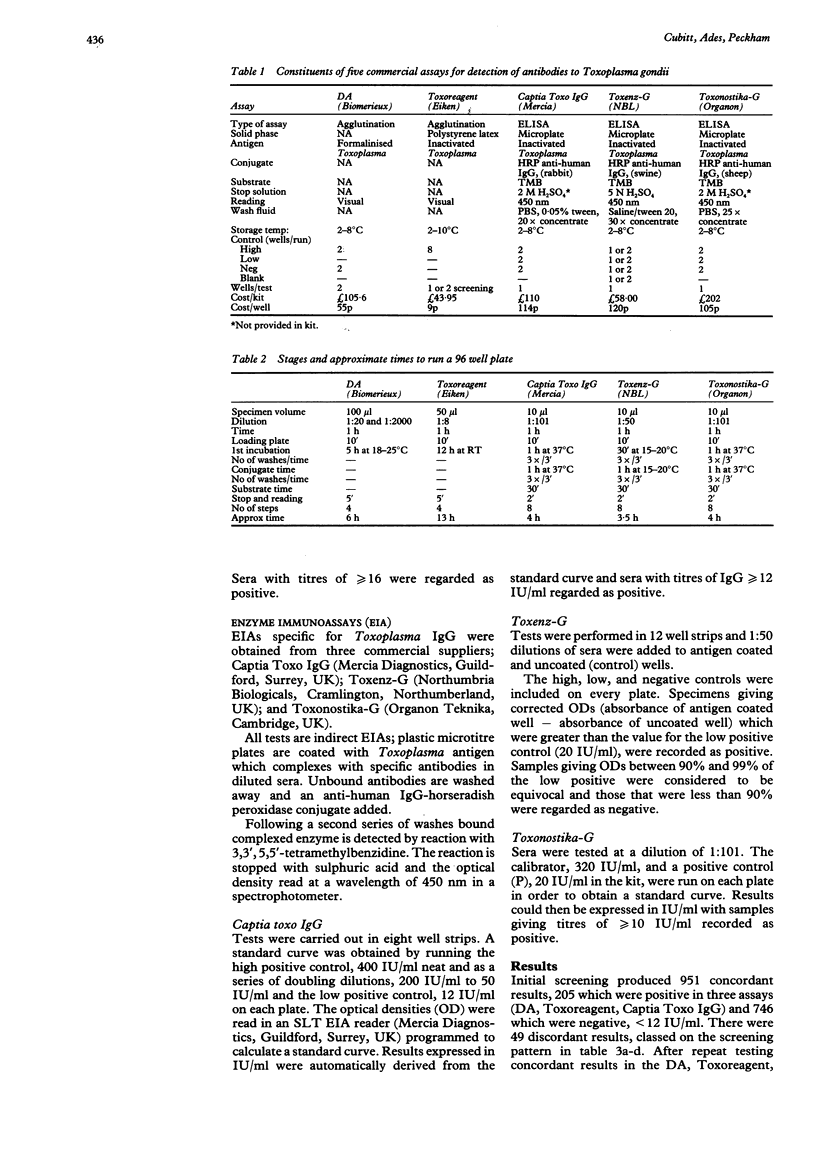
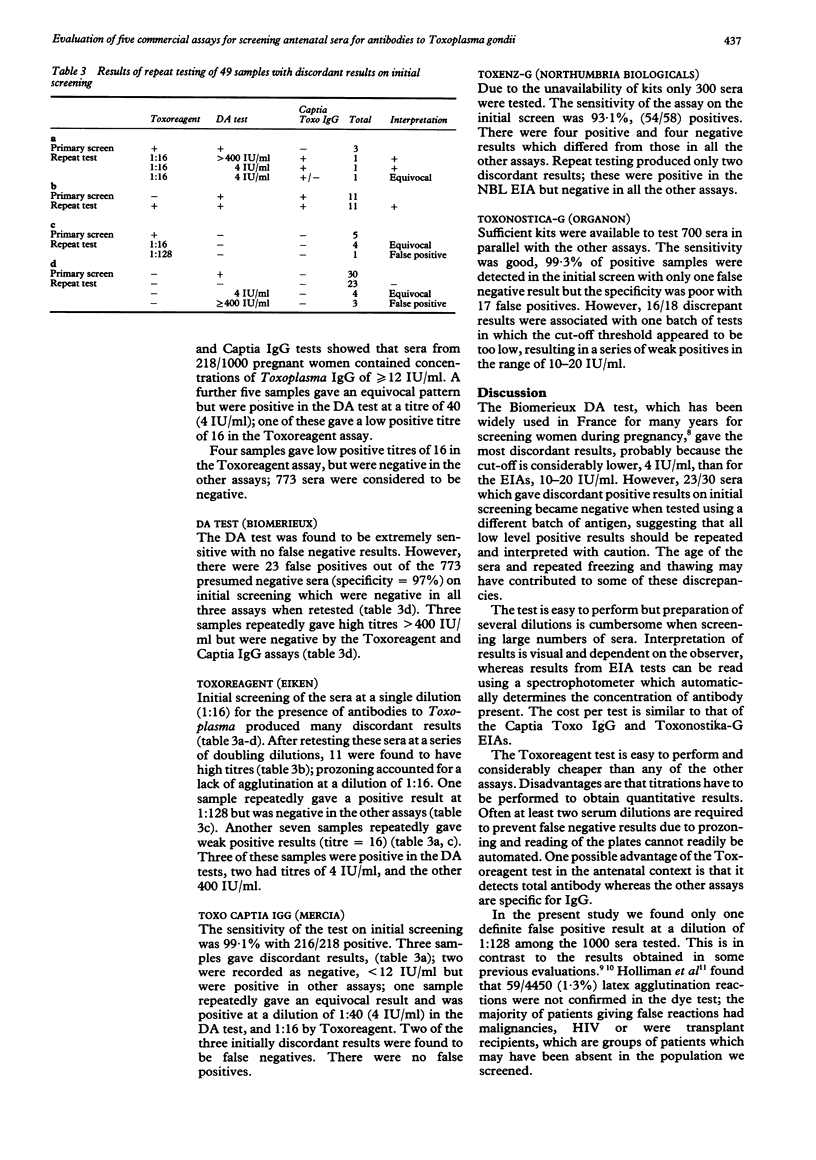
AIMS: To evaluate the suitability of five commercial assays (Toxoreagent, DA, Captia Toxo IgG, Toxenz-G, Toxonostika-G) for screening large numbers of sera for antibodies to Toxoplasma gondii. METHODS: Sera from 1000 pregnant women booking for antenatal care at a London hospital were screened in parallel by each test. Sera giving discordant results were retested. RESULTS: The Captia Toxo IgG enzyme immune assay gave the best specificity on initial screening, with 0/773 false positives and only 2/218 false negatives. The Toxoreagent latex agglutination test performed well provided sera were tested at several dilutions to prevent prozone effects; 0/218 false negatives (greater than 12 IU/ml). Only one evidently false positive result was seen in the 1000 samples tested. The DA test gave no false negative results but produced 23/773 false positives. After repeat testing there were 9/1000 sera which gave equivocal results which were negative by the Captia Toxo IgG test (less than 12 IU/ml) but with low titres of 16 in the Toxoreagent test or 4 IU/ml in the DA test. In this situation women would have been asked for a follow up sample for repeat testing. Only 300 sera were tested by Toxenz-G; initial screening produced 4/58 false negative results and 4/242 false positives. CONCLUSIONS: The Captia Toxo IgG test gave the fewest discordant results on initial screening. Results could be readily expressed in international units using a programmable plate reader, and this may be useful for epidemiological studies. The Toxoreagent test is considerably cheaper, and is a simple and reliable method for screening provided that at least two dilutions are used.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades A. E., Peckham C. S., Dale G. E., Best J. M., Jeansson S. Prevalence of antibodies to herpes simplex virus types 1 and 2 in pregnant women, and estimated rates of infection. J Epidemiol Community Health. 1989 Mar;43(1):53–60. doi: 10.1136/jech.43.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmonts G., Remington J. S. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol. 1980 Jun;11(6):562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliman R. E., Johnson J., Duffy K., New L. Discrepant toxoplasma latex agglutination test results. J Clin Pathol. 1989 Feb;42(2):200–203. doi: 10.1136/jcp.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss A. W., Chatterton J. M., Ho-Yen D. O. Congenital toxoplasmosis: to screen or not to screen? Public Health. 1990 Jan;104(1):9–20. doi: 10.1016/s0033-3506(05)80340-3. [DOI] [PubMed] [Google Scholar]
- Joynson D. H., Payne R. Screening for toxoplasma in pregnancy. Lancet. 1988 Oct 1;2(8614):795–796. doi: 10.1016/s0140-6736(88)92443-9. [DOI] [PubMed] [Google Scholar]
- Peckham C. S., Chin K. S., Coleman J. C., Henderson K., Hurley R., Preece P. M. Cytomegalovirus infection in pregnancy: preliminary findings from a prospective study. Lancet. 1983 Jun 18;1(8338):1352–1355. doi: 10.1016/s0140-6736(83)92138-4. [DOI] [PubMed] [Google Scholar]
- Wreghitt T. G., Gray J. J., Balfour A. H. Problems with serological diagnosis of Toxoplasma gondii infections in heart transplant recipients. J Clin Pathol. 1986 Oct;39(10):1135–1139. doi: 10.1136/jcp.39.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]


