ABSTRACT
Acute kidney injury (AKI) is common in hospitalised patients but is known be suboptimally managed; the National Confidential Enquiry into Patient Outcomes and Death (NCEPOD) report in 2009 identified significant failings in AKI care. An audit, using standards suggested by the NCEPOD report, of all adult inpatients with AKI in a large central-London NHS hospital in a 7-day period in 2011 showed poor recognition and management of AKI. In response, an AKI ‘care bundle’ was developed and deployed throughout the hospital along with a programme of enhanced education. Re-audit in 2013 showed that AKI was significantly more likely to have been recognised by the clinical team than in 2011, and patients with AKI were significantly more likely to have had fluid status clinically assessed and nephrotoxic medication stopped in 2013 than in 2011. There was no significant improvement in fluid administration if assessed as hypovolaemic and compliance with the guideline for prevention of contrast nephropathy. In 2011, all audit measures were met in 3.7% of patient-days versus 8.4% in 2013. More in-depth work is necessary to better understand the factors which limit optimal care.
KEYWORDS: Acute kidney injury, care bundle, audit
Introduction
Acute kidney injury (AKI) has been recognised as a serious medical problem affecting 13–18% of patients admitted to hospital. It is associated with short- and long-term complications, an increased risk of premature death and significant healthcare costs. Survivors of AKI are at risk of chronic kidney disease (CKD), including need for long-term dialysis.1–3
AKI can affect patients across all medical and surgical specialties, with only a small minority of cases being directly under the care of nephrologists.4 To date, there are no specific therapies and the emphasis is on early detection, optimisation of haemodynamics and fluid status, avoidance of nephrotoxic drugs and initiation of appropriate diagnostic investigations. In 2009, the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) identified significant failings in recognition and deficiencies in the management of AKI in hospitals in the UK.5 Care was considered to be good in fewer than 50% of cases. A further NCEPOD report in 2010 showed that a significant proportion of patients over the age of 80 years who died within 30 days of surgery had AKI.6
In 2012, Muniraju et al reported significant gaps in knowledge among junior and senior medical staff related to the prevention, diagnosis and management of AKI.7 Deficiencies were detected across all grades, and the authors called for enhanced education at undergraduate and postgraduate level.
Within the NHS in the UK there is an increasing emphasis on the prevention of avoidable harm indicating the need to develop and implement strategies to prevent AKI and its progression. Our analysis aimed to assess the impact of a hospital-wide programme (without e-alert) to improve AKI recognition and management across clinical teams, by use of an audit cycle based on standards suggested by the NCEPOD report.5
Methods
Setting
Guy's and St Thomas' NHS Foundation Hospital is a two-site 750 bed tertiary care centre in Central London admitting emergency and elective patients.
Patient selection
Adult AKI patients were identified by daily review of all inpatient blood test results from the previous days. For pragmatic reasons we decided to conduct the audit over a 7-day period (Monday morning until Monday morning). AKI was defined by the creatinine criteria of the Kidney Disease Improving Global Outcomes (KDIGO) classification, ie rise in serum creatinine by ³26.4 μmol/L in 48 hours or less, or 50% increase in serum creatinine from baseline within 7 days.8 Baseline serum creatinine was taken as the lowest value in the preceding 3 months. In case previous blood results were not available, baseline creatinine was assumed to be the upper limit of normal (ie 80 μmol/L in our laboratory). All adult inpatients were considered but patients who were on chronic dialysis, admitted to the renal or critical care department or receiving palliative care were excluded.
Audit cycle
The first audit was conducted during a 7-day period in May 2011. An audit template was created based on standards set by the NCEPOD report (Box 1). For each patient identified as having AKI, medical and nursing notes and drug charts were reviewed daily. An audit measure was judged to have been met if there was written documentation in the medical or nursing notes. As per the NCEPOD report,5 risk factors for AKI were advanced age, comorbidities, nephrotoxic drugs, hypotension, previous CKD, hypovolaemia, sepsis and poor nutritional status. Drugs with nephrotoxic potential were taken to be aminoglycosides, non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. Patients were classified as ‘vulnerable to hypovolaemia’ if they had sepsis or diarrhoea, were vomiting or ‘nil by mouth’.
Box 1.
Audit template
| > Was AKI recognised by the treating team? |
| If yes, was there a documented AKI management plan? |
| If yes, was a urine dipstick documented in the notes? |
| > Were full observations recorded? |
| > Was fluid status clinically assessed? |
| > Was fluid balance chart complete/attempted/absent? |
| > If assessed as hypovolaemic, were fluids given within 1 hour? |
| > If vulnerable to volume depletion, were maintenance fluids given? |
| > If septic, were antibiotics given promptly? |
| > If given intravenous or intra-arterial contrast, were Trust guidelines followed? |
| > If on nephrotoxic drugs, were they stopped unless absolutely necessary? |
Patients with AKI were audited and followed up daily until the end of the data collection period, AKI resolution, admission to the critical care unit, discharge from hospital or death, whichever occurred first. Data related to demographics, AKI stage, hospital outcome and medical speciality were collected. Performance against the audit measures was recorded on every day of AKI during the 7-day period. The audit team did not identify themselves to the relevant clinical teams unless there was concern that an important potentially life threatening event had not been recognised, for instance severe hyperkalaemia or toxic aminoglycoside levels.
Education and training
In response to the results of audit 1, an AKI improvement group was established in 2011, which consisted of junior and senior medical trainees, nursing staff and consultants. In addition, an AKI care bundle was developed, tested in a pilot phase and revised accordingly (Fig 1). The care bundle was launched in autumn 2012 with high impact media across the hospital, including posters on all wards and announcements on the hospital intranet and screensavers. Results of the 2011 audit and the AKI bundle were presented at the Hospital Grand Round, the Specialist Nurse Practitioner Training Day, at teaching sessions of junior medical staff and at introduction sessions to new doctors joining the trust. The bundle was available via the electronic patient record system and also in paper format. Trainees were asked to complete the bundle for every patient with AKI, to file completed forms in the medical notes and to use it as a guide for patient management. In addition, a trainee survey was used to explore the trainees' satisfaction with the training received to manage AKI and to identify learning needs.
Fig 1.
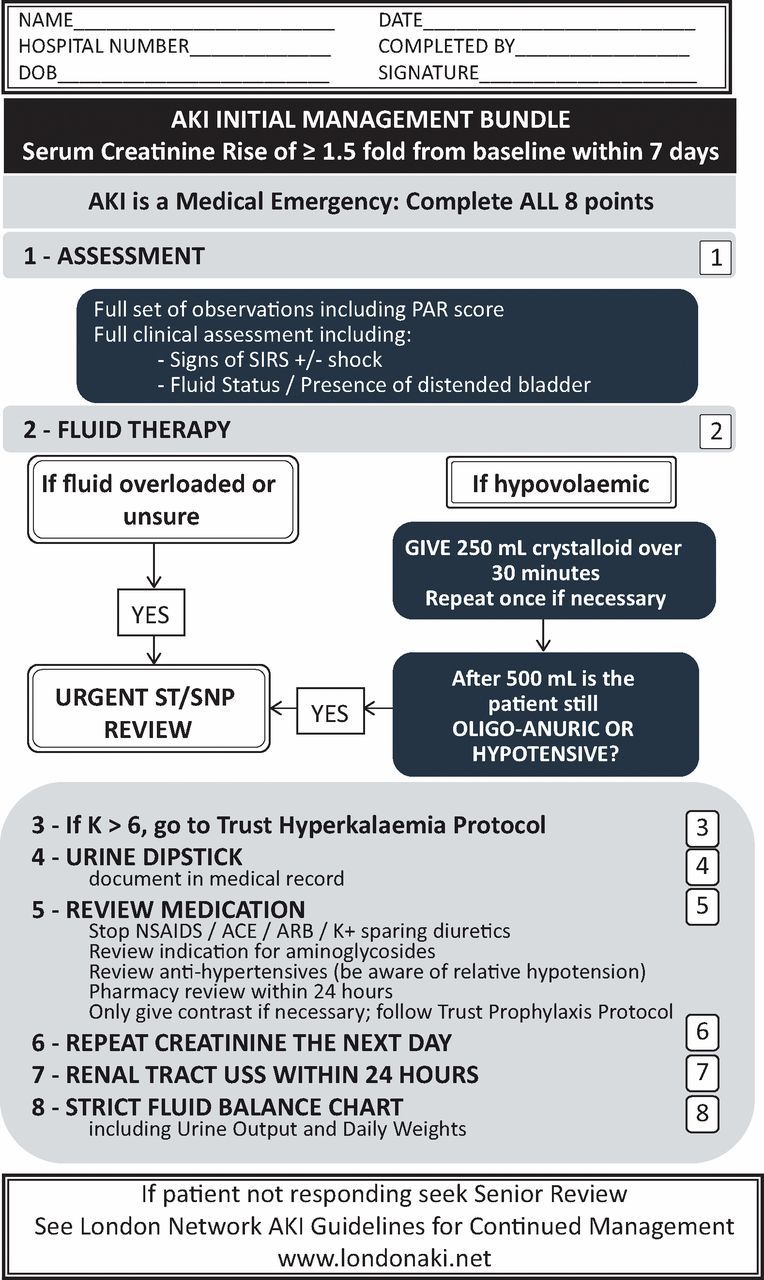
AKI Care Bundle. ACE = angiotensin-converting-enzyme; AKI = acute kidney injury; ARB = angiotensin II receptor blockers; NSAIDs = non-steroidal anti-inflammatory drugs; PAR = patient at risk; SIRS = systemic inflammatory response syndrome; USS = ultrasound scan.
In April 2013, a repeat audit was conducted over a 7-day period, under identical conditions to the first audit in 2011. The data were collected by a different group of junior doctors but the project was coordinated and supervised by the same group of senior staff.
Statistical analysis
Continuous variables were summarised as mean and standard deviation or median and interquartile range; comparison was made using the Student's t-test or Mann-Whitney U test as appropriate. Categorical variables were described as frequency (percentage); comparison was made using Pearson's Chi Squared test or Fisher's exact test as appropriate. All tests were two-sided and p<0.05 was considered statistically significant. Analysis was conducted using SPSS version 21.
Ethics
The audits and analysis had institutional approval. Need for individual informed consent was waived because this was an analysis of data collected for routine care, and there was no breach of privacy or anonymity (UK National Research Ethics Service).
Results
In audit 1 in 2011, 100 patients with AKI were identified. Data were collected on a total of 266 patient-days. On 22 of 266 days the patients were receiving end of life care and therefore only the remaining 244 days were analysed. Audit 2 in 2013 revealed 92 AKI patients with 262 patient-days available for analysis. No AKI patient was receiving palliative care during the audit period in 2013. Baseline characteristics of both patient cohorts were similar, although the 2013 cohort had more risk factors for AKI (Table 1).
Table 1.
Baseline characteristics of patients with AKI.
| Characteristics | 2011 (n = 100) | 2013 (n = 92) | p value |
|---|---|---|---|
| Mean age, years (SD) | 71.9 (18.7) | 68.5 (17.1) | 0.20 |
| Male gender, n (%) | 54 (54) | 47 (51) | 0.69 |
| Mean baseline creatinine where known, μmol/L (SD) | 112.2 (88.9) | 101.1 (60.0) | 0.38 |
| Admitted under medical specialty, n (%) | 63 (63) | 58 (63) | 1 |
| Admitted under surgical specialty, n (%) | 35 (35) | 34 (37) | 0.78 |
| Median AKI risk factors, n (IQR) | 2 (1–2) | 2 (1–3) | 0.007 |
AKI = acute kidney injury; IQR = interquartile range; SD = standard deviation.
Management of AKI
In 2013 there was a significant improvement in recognition of AKI by the clinical team compared to 2011, from 59% to 79% (Table 2). Assessment of fluid status, completion of fluid balance chart and discontinuation of nephrotoxic medications also improved significantly. There was no significant improvement in either fluid administration if assessed as hypovolaemic, or in antibiotic administration within one hour in the case of sepsis, and compliance with the hospital guideline for prevention of contrast nephropathy did not increase.
Table 2.
Univariate analysis of AKI recognition and management based on AKI patient-days.
| Quality indicator | 2011a | 2013a | p value |
|---|---|---|---|
| AKI recognised by treating team (%) | 144/244 (59) | 197/262 (75) | <0.001 |
| If recognised, AKI management plan documented in medical notes (%) | 118/144 (82) | 167/197 (85) | 0.49 |
| Full set of observations recorded (%) | 240/244 (98) | 256/262 (98) | 1 |
| Fluid status clinically assessed and documented in medical notes (%) | 91/244 (37) | 170/262 (65) | <0.001 |
| Fluid balance chart completed (%) | 77/244 (32) | 117/262 (45) | 0.002 |
| Fluids given if assessed as hypovolaemic (%) | 28/35 (80) | 60/75 (80) | 1 |
| Maintenance fluids given if vulnerable to volume depletion (%) | 99/121 (82) | 129/185 (70) | <0.001 |
| Antibiotics given within one hour of diagnosis of sepsis (%) | 20/24 (83) | 23/28 (82) | 1 |
| Hospital guideline for contrast nephropathy prophylaxis followed if receiving contrast (%) | 8/16 (50) | 5/9 (56) | 1 |
| Potentially nephrotoxic medications stopped if relevant (%) | 27/101 (27) | 68/111 (61) | <0.001 |
afigures refer to the number of days when the criterion was met relative to the number of days when the criterion ideally should have been fulfilled. AKI = acute kidney injury.
In 2011, the audit team intervened on 3 occasions during the 7-day audit period and informed the medical team about serious potentially life-threatening complications of AKI (severe hyperkalaemia, development of AKI stage III and non-discontinuation of nephrotoxic drugs). In 2013, the team intervened on 4 occasions.
In 73 patients, the first day of AKI (d1-AKI) fell within the 2011 data collection period, compared to 68 in 2013. 22% of patients had AKI stage II or III as per KDIGO classification on the first day of AKI in 2013 compared to 8% in 2011 (p = 0.02). Analysis of care on d1-AKI showed that there was significant improvement in 2013 compared to 2011 in recognition of AKI by the treating team (51 vs 68%) and discontinuation of nephrotoxic medications (29 vs 73%) (Table 3). Other parameters did not improve significantly.
Table 3.
Univariate analysis of AKI recognition and management on day when criteria for AKI were met for first time.
| Quality indicator | 2011a | 2013a | p value |
|---|---|---|---|
| AKI recognised by treating clinical team (%) | 37/73 (51) | 46/68 (68) | 0.04 |
| If recognised, urine dipstick result documented in notes (%) | 18/37 (49) | 23/46 (50) | 1 |
| If recognised, AKI management plan documented in medical notes (%) | 31/37 (84) | 40/46 (87) | 0.68 |
| Full set of observations recorded (%) | 69/73 (95) | 67/68 (99) | 0.37 |
| Fluid status clinically assessed and documented in medical notes (%) | 31/73 (42) | 40/68 (59) | 0.052 |
| Fluid balance chart completed (%) | 18/73 (25) | 23/68 (34) | 0.23 |
| Fluids given if assessed as hypovolaemic (%) | 14/19 (74) | 14/22 (64) | 0.49 |
| Maintenance fluids given if vulnerable to hypovolaemia (%) | 35/45 (78) | 35/50 (70) | 0.39 |
| Antibiotics given within one hour of diagnosis of sepsis (%) | 13/15 (87) | 10/14 (71) | 0.39 |
| Hospital guideline for contrast nephropathy prophylaxis followed if receiving contrast (%) | 5/9 (56) | 4/8 (50) | 1 |
| Potentially nephrotoxic medications stopped if possible (%) | 11/37 (29) | 45/62 (73) | <0.001 |
adata indicate the number of patients in whom the criteria were fulfilled relative to the number of patients to whom the criterion applied. AKI = acute kidney injury.
In 2013, all audit measures were met in 8.4% of patient-days versus 3.7% in 2011. There was no difference in performance between medical and surgical clinical teams. Across both years, there was no significant improvement in recognition of AKI based on severity of AKI, but there was an association between recognition of AKI and improvement in some performance criteria (Table 4).
Table 4.
Univariate analysis of AKI management stratified by AKI recognition on AKI patient-days (2011 and 2013 data combined).
| Quality indicator | AKI recognised by treating clinical teama | AKI not recognised by treating clinical teama | p value |
|---|---|---|---|
| Full set of observations recorded (%) | 337/341 (99) | 159/165 (96) | 0.09 |
| Fluid status clinically assessed (%) | 215/341 (63) | 46/165 (28) | <0.001 |
| Fluid balance chart completed (%) | 143/341 (33) | 51/165 (31) | 0.02 |
| Fluids given if assessed as hypovolaemic (%) | 74/92 (80) | 14/18 (78) | 1 |
| Maintenance fluids given if vulnerable to hypovolaemia (%) | 175/221 (79) | 53/84 (63) | 0.004 |
| Antibiotics given within one hour of diagnosis of sepsis (%) | 30/36 (83) | 13/16 (81) | 1 |
| Hospital guideline for contrast nephropathy prophylaxis followed if receiving contrast (%) | 7/14 (50) | 6/11 (55) | 1 |
| Potentially nephrotoxic medications stopped if relevant (%) | 82/127 (65) | 13/85 (15) | <0.001 |
avalue/applicable patient-days (%). AKI = acute kidney injury.
Outcomes
Hospital mortality of AKI patients enrolled in audit 1 (2011) was 12% compared to 10% in AKI patients in audit 2 (2013). There was no difference in hospital outcome between patients in whom AKI was recognised by the clinical team on the first day of AKI compared to those in whom AKI was not immediately recognised.
The proportion of audited patients with AKI mentioned in their hospital discharge summary rose from 38% to 55% between 2011 and 2013.
Results of trainee survey
The survey was completed by 68 trainees of all grades (foundation doctors to specialist trainees in final year of training). 37% of participants were trainees in acute general medicine and 15% were from surgical specialties. The remaining participants were trainees in subspecialties. Although 92% stated that they were comfortable managing patients with AKI, 96% of respondents felt that there was a need for better undergraduate and postgraduate training in AKI. The preferred mode of teaching was ‘on the job training’ and ‘case-based discussions’.
Discussion
Our study shows that the implementation of an AKI bundle combined with an enhanced teaching programme can improve recognition of AKI and some aspects of AKI care, but does not achieve optimal management in all cases.
AKI is usually asymptomatic until renal function ceases completely. It is often found in conjunction with other acute medical problems and not always preventable. AKI is increasing in incidence, likely in part due to increasing comorbidities.9
There are no characteristic clinical signs and the diagnosis is based on an increase in serum creatinine or fall in urine output, or both.8 As a result, the development of AKI may not be recognised immediately by clinical teams, especially if other competing urgent clinical demands coexist. The management of AKI consists of attention to detail with particular focus on optimisation of fluid and haemodynamic status, avoidance of additional nephrotoxic insults, and early recognition of an underlying renal aetiology. It is hoped that these strategies, if applied early, reverse the acute process and prevent progression to acute renal failure.
A care bundle is a structured way of improving the processes of care and patient outcomes through a small set of evidence-based practices that when performed collectively and reliably, improve patient outcomes.10 When a bundle element is missed, the patient is at much greater risk of complications. Care bundles have been successfully integrated into the management of potentially life-threatening conditions like sepsis or ventilator-associated pneumonia.11
Finlay et al identified several risk factors associated with community-acquired AKI in undifferentiated acute medical patients and proposed that an AKI bundle could be applied to high-risk patients in order to prevent AKI occurring.12 Tsui et al reported that the introduction of a specially designed care bundle in a large urban London hospital significantly improved assessment and optimisation of fluid status, performance of urinalysis, discontinuation of nephrotoxic drugs, appropriate monitoring of urine output and prescription of renal drug doses.13 Our analysis shows that a care bundle in combination with enhanced education improved some aspects of AKI management but important gaps remained. Unsurprisingly, recognition of AKI was associated with other care quality measures being met. We also found that an AKI bundle was used more often in patients with AKI on admission to hospital compared to patients who developed AKI later while in hospital. Since acquired AKI usually occurs in the context of general deterioration or as a result of iatrogenic contributions, it is possible that other more urgent needs distracted the clinical teams from recognising AKI. In this situation, AKI may have been given a low priority amid numerous other clinical demands. Although we did not find a significant difference, a larger study that also looked at all cases of AKI within a large UK hospital, and with comparable rates of AKI recognition and some management parameters, found a significantly higher mortality rate in ‘hospital-acquired’ than ‘community-acquired’ AKI.14
Clearly, more in-depth work is necessary to better understand the factors that may have contributed to the lack of improvement in all areas and limited optimal management of all AKI patients. There are several potential reasons: in the UK, there is a high staff turnover of junior medical staff, especially in larger teaching hospitals. Education sessions will have to take this into account, and ideally, AKI teaching should be integrated into a rolling programme. Since the management of AKI involves different healthcare professions, teaching sessions need to reflect this. Simulation training may be one tool to improve knowledge and clinical practice of multidisciplinary teams. There is also a need to incorporate AKI education into the curriculum at medical school.15 Other potential factors which may have contributed to delayed recognition and failure to apply the care bundle are shiftworking of junior medical staff and transfer of patients between clinical specialties and wards.
It is important to acknowledge some limitations of our project. First, the audit cycle was performed in a large single centre with a high turnover of medical and nursing staff. Therefore, our conclusions may not be generalisable. We acknowledge that other studies have shown better results.12 Second, the data in 2011 and 2013 were collected by 2 different groups of junior doctors. Although they used the same audit template and were supervised by the same team, it is possible that entries in the medical notes were interpreted differently. Third, we collected data over a 7-day period and acknowledge that a longer period may have avoided potential confounding factors. Finally, we only used documented entries in the medical or nursing notes as evidence that the individual audit measures had been met. It is possible that AKI management was indeed better than documented in writing.
Phillips et al showed that the recognition of patients with AKI is not only difficult in hospitals in the UK but a worldwide problem. 16 For instance, data from 3 large teaching hospitals in Ethiopia confirm great variability in the extent to which doctors recognised patients at high risk of AKI.
Addressing the identified gaps will require organisational changes in addition to sustained educational efforts. Focusing on teaching and training is not sufficient, especially if conducted at formal teaching sessions or meetings, rather than ‘at the bedside’ and ‘on the job’. Additional measures to improve the care of patients with AKI include better handover procedures between changing shifts and specialties and improved documentation in the medical notes. These measures need to include all relevant healthcare professionals. Senior nurse and ward managers have a particularly important role in generating and maintaining change and introducing new quality standards. In some hospitals, nurse-led AKI outreach teams, based on the model of critical care outreach services, and AKI specialist nurses have also been introduced with promising results.17 Finally, it is hoped that innovative solutions like electronic alerting and clinical decision support systems will help to identify AKI patients early and protect them from any remaining deficiencies in knowledge or practice. In England, a recent National Patient Safety Alert by NHS England has mandated the use of a detection algorithm for AKI in all acute hospitals. It is hoped that earlier identification of patients with AKI will translate into earlier intervention and improved outcomes. The first randomised controlled trial to examine the effect of an AKI e-alert showed no difference in maximum increase in serum creatinine, need for dialysis or mortality.18 Our hospital is currently in the process of implementing an e-alert via the electronic patient record system. Future audits and evaluations will inform whether this initiative, in combination with ongoing educational projects and changes in clinical practice, has a role in improving the management and outcome of AKI.
Acknowledgements
The authors would like to thank the AKI Improvement Group at Guy's and St Thomas' Hospital, the London AKI Network and those who assisted with data collection: H Wilkinson, S Henderson, A Irvine, D Bangaru-Raju, B Freudenthal, T Sanctuary, A Buckingham, T Ellimah, C Junghans Minton, C Masterton-Smith, S Rossi, J Sutherby, KES Warren and MA Yates.
This work was presented at the 2013 Meeting of the American Society of Nephrology, the 2013 Meeting of the South West and East Kidney Society and the 2014 Annual NCEPOD meeting.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaFrance JP, Miller DR. Acute kidney injury associated with increased long-term mortality. J Am Soc Nephrol 2010; 21:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham KA, Thompson EB, Bodger K, Pearson M. Inequalities in outcomes of acute kidney injury in England. QJM 2012;105:729–40. [DOI] [PubMed] [Google Scholar]
- 5.National Confidential Enquiries into Patient Outcome and Death (NCEPOD) Adding insult to injury. A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury. London: NCEPOD, 2009. [Google Scholar]
- 6.National Confidential Enquiry into Patient Outcome and Death (NCEPOD) Elective and emergency surgery in the elderly an age old problem. London: NCEPOD, 2010. [Google Scholar]
- 7.Muniraju TM, Lillicrap MH, Horrocks JL, et al. Diagnosis and management of acute kidney injury: deficiencies in the knowledge base of non-specialist, trainee medical staff. Clin Med 2012;12:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 9.Siew ED, Davenport A. Growth of acute kidney injury. Kidney Int 2015;87:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Healthcare Improvement Evidence-based care bundles. Cambridge, MA: IHI, 2015. Available online at www.ihi.org/topics/bundles/Pages/default.aspx [Accessed 30 July 2015]. [Google Scholar]
- 11.Morris AC, Hay AW, Swann DG, et al. Reducing ventilator–associated pneumonia in intensive care: Impact of implementing a care bundle. Crit Care Med 2011;39:2218–24. [DOI] [PubMed] [Google Scholar]
- 12.Finlay S, Bray B, Lewington AJ, et al. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med 2013;13:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsui A, Rajani C, Doshi R, et al. Improving recognition and management of acute kidney injury. Acute Med 2014;13:108–12. [PubMed] [Google Scholar]
- 14.Aitken E, Carruthers C, Gall L, et al. Acute kidney injury: outcomes and quality of care. QJM 2013;106:323–32. [DOI] [PubMed] [Google Scholar]
- 15.Ali MN, Lewington AJ. Invited manuscript poster on renal-related education American Society of Nephrology, Nov. 16–21, 2010. Do medical trainees receive adequate training in the management of acute kidney injury? Ren Fail 2011;33:669–71. [DOI] [PubMed] [Google Scholar]
- 16.Phillips LA, Allen N, Phillips B, et al. Acute kidney injury risk factor recognition in three teaching hospitals in Ethiopia. S Afr Med J 2013;103:413–8. [DOI] [PubMed] [Google Scholar]
- 17.Thomas ME, Sitch A, Baharani J, Dowswell G. Earlier intervention for acute kidney injury: evaluation of an outreach service and a long-term follow-up. Nephrol Dial Transplant 2015;30:239–44. [DOI] [PubMed] [Google Scholar]
- 18.Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury; a single-blind, parallel-group randomised controlled trial. Lancet 2015;25 February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


