ABSTRACT
Acute liver failure (ALF) is a rare critical illness with high mortality whose successful management requires early recognition and effective initial management. Though it may result from a wide variety of causes, in the UK and much of the developed world most cases result from paracetamol-induced hepatotoxicity, and administration of antidotal N-acetyl cysteine at first recognition is key. Involvement of local critical care services should occur at an early stage for stabilisation, monitoring and supportive care with parallel discussion with specialist liver centres to identify those patients who may benefit from transfer. Prognostic criteria are applied to identify patients for emergency liver transplantation, and candidates for surgery are prioritised on waitlisting schemes. Outcomes now approach that of elective surgery. However, the majority of cases, and particularly those with paracetamol-induced disease, recover with supportive medical care alone. Overall outcomes for patients with ALF have improved dramatically over the last three decades, but mortality remains unacceptable and further advances in care are required.
KEYWORDS: Acute liver failure, liver transplantation, encephalopathy, paracetamol, critical care
Key points
Early identification of acute liver failure (ALF) is key to optimise management, identify candidates for urgent liver transplantation and improve survival.
The majority of cases of ALF in the UK are due to paracetamol-induced hepatotoxicity; therapy with N-acetylcysteine should be commenced immediately on identification.
Routine correction of coagulopathy with blood products is seldom necessary as spontaneous bleeding is uncommon in ALF and evaluation of coagulopathy is central to assessing prognosis.
Risk of sepsis is high and can precipitate hepatic encephalopathy and multi-organ failure, which can preclude successful transplantation; a low threshold for administration of antibiotics is maintained.
Further research into pathophysiology of ALF and optimising hepatic regeneration are required.
Introduction
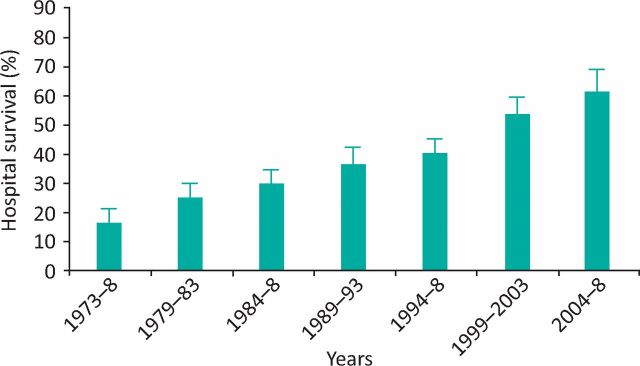
Acute liver failure (ALF) is a rare, life-threatening condition characterised by rapid evolution from deranged liver function to coagulopathy and encephalopathy, occurring most commonly in younger adults without pre-existing liver disease. Though advances in critical care and utilisation of liver transplantation (LT) have improved survival in recent years (Fig 1), mortality remains high.1,2 Clinical management of ALF presents unique challenges, as its rarity, severity and heterogeneity have limited clinical trials and the evidence base to guide management is restricted.
Fig 1.
Hospital survival in 2095 ALF admissions to the Liver Intensive Therapy Unit, King's College Hospital by era. Error bars represent 95% confidence intervals (p<0.00001). Reproduced with permission.2 ALF = acute liver failure.
Presentation
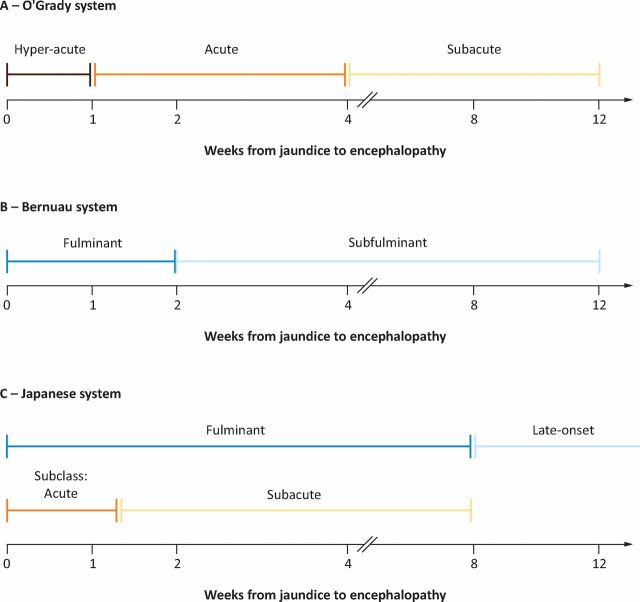
ALF is often defined according to the time interval from the development of jaundice to onset of hepatic encephalopathy (HE). By example, the commonly utilised O'Grady classification categorises ALF as hyperacute, acute or subacute (Fig 2).3,4 This classification is of prognostic value, as those with a hyperacute presentation, in whom coagulopathy and encephalopathy are most severe, paradoxically have the highest rates of survival with medical management alone. Hyperacute ALF commonly follows paracetamol-induced hepatotoxicity or acute viral infection. In contrast, those presenting with subacute ALF, with less dramatic coagulopathy and encephalopathy, have consistently higher mortality without LT. These cases are often due to idiosyncratic drug reactions and may be confused for chronic liver disease.
Fig 2.
Classification systems for ALF. The late onset period runs from 8 to 24 weeks in the Japanese system. Reproduced with permission.4 ALF = acute liver failure.
Aetiology
The major cause of ALF in the developed world is drug-induced liver injury (DILI).5 In Europe and the United States, this is most commonly due to paracetamol.6 Mortality rates are highest in those who stagger ingestion and delay presentation to medical attention. In all cases, prompt recognition and treatment is vital to improve survival. Idiosyncratic DILI is uncommon, even after exposure to hepatotoxic drugs, and few patients with DILI progress to ALF; however when they do, mortality may be high.7
The majority of ALF cases in the developing world are due to acute viral infections from hepatitis A, B and E. Hepatitis A and E spread via faeco–oral transmission and cause self-limiting illness, which rarely progresses to ALF. Hepatitis B is transmitted via contact with infected bodily fluids. In the developed world, hepatitis B most commonly causes ALF when the virus is ‘reactivated’ in chronic carriers, rather than in de novo infection. Reactivation occurs in the context of immunosuppressive therapy, such as biologics, and is preventable with prophylactic antiviral therapy. Patients should be screened for hepatitis B, including core antibody, prior to immunosuppressive drug exposure.8 Rare viral causes of ALF include herpes simplex virus, cytomegalovirus (CMV), Epstein–Barr virus, adenovirus and parvoviruses.
Ischaemic liver injury, such as may occur in critically ill individuals with circulatory failure or septic shock, can cause significant hepatic necrosis and major but transient elevation of serum transaminases. Occurring most commonly after significant hypotension or hepatic congestion, its treatment addresses the underlying cause.
Rare causes of ALF include metabolic diseases, such as Wilson's, characterised by hyperbilirubinaemia, low alkaline phosphatase and Coomb's negative haemolysis. This has extremely high mortality without LT. Other rare causes of ALF include malignant infiltration, Budd–Chiari syndrome and heatshock. ALF can occur in pregnancy where causes include acute fatty liver of pregnancy and haemolysis, elevated liver enzymes and low platelets (HELLP syndrome). In most such cases, prognosis is good and symptoms improve after delivery. Their management should be undertaken in an institution with experience in both liver and neonatal care.
Despite extensive investigation, the aetiology of ALF remains unknown in up to 20% of cases.5 1 summarises initial investigations to be performed in patients with suspected ALF.9
Box 1.
Initial investigations for patients presenting with ALF. Adapted with permission.9
| > Arterial blood gases: include lactate and arterial ammonia |
| > Haematology: full blood count and coagulation studies (INR and prothrombin time, fibrinogen); blood group |
| > Biochemistry: liver function tests (AST, ALT, ALP, GGT, bilirubin, albumin), renal function and electrolytes (Na, K, urea, creatinine, Mg, PO4), glucose, amylase, thyroid function tests, creatine kinase and lipids |
| > Hepatitis serology and viral screen: anti-HAV IgM, HBsAg, anti-HB core IgM and anti-HEV IgM, EBV, CMV, HSV, HIV, adenovirus and parvovirus |
| > Autoantibodies: antinuclear, anti-smooth muscle actin, anti-liver/kidney microsomal antibodies and immunoglobulins |
| > Toxicology screen: paracetamol and salicylate levels |
| > Metabolic screen: serum copper and caeruloplasmin |
| > Liver ultrasound: include assessment of patency of hepatic and portal veins and hepatic artery |
ALF = acute liver failure; ALP = alkaline phosphatase; ALT = alanine transaminase; AST = aspartate aminotransferase; CMV = cytomegalovirus; EBV = Epstein–Barr virus; GGT = gamma-glutamyl transferase; HAV = hepatitis A virus; HB = hepatitis B; HEV = hepatitis E virus; HIV = human immunodeficiency virus; HSV = herpes simplex virus; IgM = immunoglobulin M; INR = international normalised ratio; K = potassium; Mg = magnesium;
Na = sodium; PO4 = phosphate.
Management
Initial measures
ALF must be recognised early, with subsequent care occurring in a critical care setting. Presentation with confusion or agitation is seen with hyperacute disease and may cause diagnostic delay, but liver biochemical testing is diagnostic. Subacute ALF can easily be mistaken for chronic liver failure. Early contact with a specialist liver centre is key to optimise management and identify those who may require LT, to facilitate safe and expeditious transfer.
Clinical management seeks to identify and treat precipitants, while optimising conditions for liver regeneration and preventing sepsis and other complications. Continuous prognostic assessment is performed, such that waitlisting for LT can be performed at an early stage.
Specific therapies
In paracetamol-induced hepatotoxicity, N-acetylcysteine (NAC) is an effective treatment, although efficacy is closely related to the interval between drug ingestion and commencement of therapy.10 NAC is also beneficial in non-paracetamol-induced ALF, improving survival in patients with low-grade encephalopathy.11 Treatment with NAC is continued until synthetic function is improved or transplantation is performed. If a viral cause is identified, specific antiviral therapy, such as ganciclovir for CMV, may be beneficial. Autoimmune hepatitis may present as ALF, and treatment with intravenous corticosteroids is often commenced. However, this increases sepsis risk and may preclude definitive LT. One study suggested that in cases of ALF due to immune-mediated pathologies, corticosteroids did not improve survival.12 The majority of these patients are likely to have liver injury too advanced for steroid rescue, and early LT should be considered.
Ongoing care
Progression to HE can occur rapidly, particularly in those with hyperacute disease. Patients progressing to agitation, drowsiness or coma should undergo early intubation to secure the airway and facilitate control of gas exchange. Patients with ALF are functionally immunosuppressed and at high risk of nosocomial infection. Empirical antibiotic therapy is recommended for those with encephalopathy, organ failure, coagulopathy and those whose illness is likely to progress.13 Despite markedly abnormal standard tests of coagulation, spontaneous bleeding is very uncommon. Functional assays have demonstrated no bleeding tendency and many patients may be pro-thrombotic, reflecting a balanced loss of pro- and anti-coagulant factors.14 Serial evaluation of coagulation variables is central to prognostic evaluation and routine correction with clotting products is not recommended.
Complications
Cardiorespiratory
Hypotension and circulatory instability are common in ALF. Cardiovascular support does not differ from other critical illnesses and requires correction of circulating volume, systemic perfusion and oxygen delivery. Patients are often hypovolaemic at presentation and if hypotension persists after adequate volume resuscitation, vasopressors are administered. Functional adrenocortical insufficiency can occur in ALF and intravenous glucocorticoids should be considered in those unresponsive to fluid repletion and vasopressors. With early intubation, respiratory failure is unusual in the initial stages of ALF but may develop later from nosocomial infection. Management is again similar to other critical illnesses, but the effects of carbon dioxide concentration on the cerebral circulation mandate its careful control.
Renal
Acute kidney injury is common in ALF, and is associated with increased mortality.15 Causes are multifactorial and include acute tubular necrosis and drug toxicity; nephrotoxic drugs are stopped and haemodynamic status optimised. If renal replacement therapy (RRT) is required, continuous modes are preferred to achieve more stable haemodynamics. Novel indications for RRT include control of hyperammonaemia and lactic acidosis.
Neurological
Severe HE in ALF is a poor prognostic indicator associated with risk of cerebral oedema (CO) and intracranial hypertension, though for reasons that are not fully understood, this complication is becoming uncommon. The goal of clinical management is to prevent HE, and when it develops to limit its severity and avoid CO. HE is thought to result from the cerebral effects of high concentrations of circulating neurotoxins and permissive effects of the systemic inflammatory response. Strategies to treat HE in chronic liver disease, such as non-absorbable antibiotics and lactulose, are inappropriate in ALF. Care targets the prevention of infection, control of circulating ammonia concentration, and maintenance of stable cerebral perfusion and metabolism. This is most often achieved with a combination of sedation, avoidance of fever and RRT.
Metabolic
Patients with ALF are at high risk of hypoglycaemia, which is preventable with intravenous glucose infusion. Large volume infusion of hypotonic fluids is avoided as it risks hyponatraemia and increases the risk of CO. Nutritional support is essential in highly catabolic patients with ALF but monitoring of blood ammonia concentration is required to allow transient reduction in protein load if necessary.
Transplantation
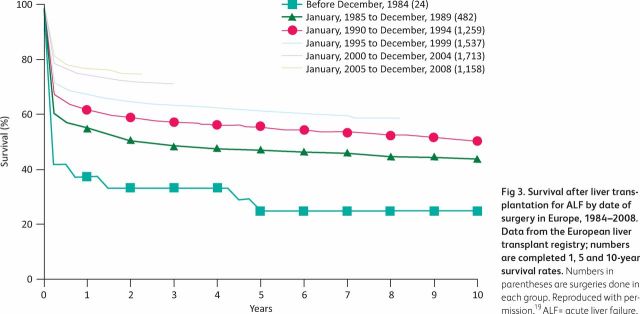
The identification of patients unlikely to survive with medical therapy alone at an early stage of illness allows the identification of potential candidates for LT. Multiple prognostic evaluation systems have been developed, but the King's College Criteria are the best characterised and form the basis of the super-urgent listing criteria in the UK.16,17 Recognising severity of illness, waitlisting schemes prioritise patients with ALF and the majority who are listed are transplanted. With improvements in care over time (Fig 3), survival in those transplanted with ALF now approaches that of elective LT, with most deaths occurring due to infection in the first three months post-LT.18,19
Fig 3.
Survival after liver transplantation for ALF by date of surgery in Europe, 1984–2008. Data from the European liver transplant registry; numbers are completed 1, 5 and 10-year survival rates. Numbers in parentheses are surgeries done in each group. Reproduced with permission.19 ALF = acute liver failure.
Alternative therapies
The limited availability of LT and the need to stabilise urgently waitlisted patients has led to interest in the development of artificial liver support devices. The most well known is the molecular adsorbent recirculating system (MARS), which uses albumin dialysis to extract toxins normally processed by the liver. Although some case series suggested clinical and biochemical improvements, a multicentre randomised controlled trial of MARS against conventional management in ALF did not demonstrate any survival benefit.20 Hepatocyte transplantation has been used successfully in children with inborn errors of metabolism but its use is limited. Initial reports suggest that high-volume plasma exchange may be beneficial in selected patients. For all such strategies, use is currently limited to clinical trials and further research is needed.
Conclusion
ALF is a rare and rapidly evolving clinical condition requiring early identification and critical care management. Earlier application of specific therapies, advances in support of multi-organ dysfunction, and the selective use of LT have led to improved survival. Better understanding of the pathophysiology of ALF, and optimal conditions required for hepatic regeneration, will likely result in further improvements.
References
- 1.Escorsell A, Mas A, de la Mata M, Spanish Group for the Study of Acute Liver Failure Acute liver failure in Spain: analysis of 267 cases. Liver Transpl 2007;13:1389–95. [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol 2013;59:74–80. [DOI] [PubMed] [Google Scholar]
- 3.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342:273–5. [DOI] [PubMed] [Google Scholar]
- 4.Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525–34. [DOI] [PubMed] [Google Scholar]
- 5.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947–54. [DOI] [PubMed] [Google Scholar]
- 6.Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010;52:2065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008;135:1924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nature Rev Clin Oncol 2012;9:156–66. [DOI] [PubMed] [Google Scholar]
- 9.Singanayagam A, Bernal W. Update on acute liver failure. Curr Opin Crit Care 2015;21:134–41. [DOI] [PubMed] [Google Scholar]
- 10.Craig DG, Bates CM, Davidson JS, et al. Overdose pattern and outcome in paracetamol-induced acute severe hepatotoxicity. Br J Clin Pharmacol 2011;71:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology 2014;59:612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karvellas CJ, Cavazos J, Battenhouse H, et al. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol 2014;12:1942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stravitz RT, Lisman T, Luketic VA, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol 2012;56:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tujios SR, Hynan LS, Vazquez MA, et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol 2015;13:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McPhail MJ, Wendon JA, Bernal W. Meta-analysis of performance of Kings's College Hospital Criteria in prediction of outcome in non-paracetamol-induced acute liver failure. J Hepatol 2010;53:492–9. [DOI] [PubMed] [Google Scholar]
- 17.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;97:439–45. [DOI] [PubMed] [Google Scholar]
- 18.Germani G, Theocharidou E, Adam R, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol 2012;57:288–96. [DOI] [PubMed] [Google Scholar]
- 19.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010;376:190–201. [DOI] [PubMed] [Google Scholar]
- 20.Saliba F, Camus C, Durand F, et al. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med 2013;159:522–31. [DOI] [PubMed] [Google Scholar]