Abstract
Increasing technological advances have resulted in the recognition of a range of genetic conditions not traditionally seen by clinical genetics teams. This has implications for the education of other healthcare professionals who may have insufficient knowledge to identify or support families with these conditions. The national genetic diabetes nurse (GDN) project, which trains diabetes specialist nurses (DSNs), was started in 2002 to increase awareness of monogenic diabetes among healthcare professionals across the UK. This paper describes the development and evaluation of the first 10 years of this project, indicating that GDNs have increased diagnostic referral rates and supported local families through diagnosis and treatment changes across the UK. The GDN project has proved an effective, innovative means of disseminating new genetic information from a centre of excellence and is suggested as a model for the successful and rapid dissemination of genetic information into routine clinical care in other conditions
KEYWORDS : Genetic diabetes nurses, monogenic diabetes, maturity-onset diabetes of the young (MODY), service provision
Introduction
Genetic causes of a range of conditions not traditionally seen by clinical geneticists are increasingly being identified. As these patients are treated across a variety of health departments, ‘new’ genetic services need to be developed and integrated into mainstream medical care.1 The National Health Service (NHS) national genetics education centre recognised the need to raise awareness of genomics among health professionals in general, while providing in-depth knowledge for specialists.2 However, the care of patients managed outside clinical genetics service teams currently remains highly inequitable as a result of limited awareness of genetic conditions.
Monogenic diabetes accounts for 2–3% of diabetes in the UK,3,4 but remains poorly recognised despite the identification and clinical characterisation of specific subtypes caused by mutations in single genes. Patients are misdiagnosed as having type 1 or type 2 diabetes in around 80% of cases5 and are consequently inappropriately treated. Ensuring the correct diagnosis is important because most can be successfully treated with tablets even after years of insulin injections.6,7 Similar issues have been identified in other conditions: familial hypercholesterolaemia remains undiagnosed in approximately 85% of cases8 and, in inherited cardiovascular conditions, a 10- to 20-fold variation in referral and genetic testing rates between different UK regions has been reported.9 Effective mechanisms are therefore required to ensure that affected individuals have access to testing and specialist services,10 and the need for education of professionals involved in the care of individuals with such genetic conditions is clear.
The monogenic diabetes team at the Royal Devon and Exeter NHS Foundation Trust and University of Exeter Medical School has led research into monogenic diabetes since 1995 and has established diagnostic and clinical services with a UK-wide NHS genetic testing service in 2000 (Fig 1). In the UK, 483 cases of monogenic diabetes had been confirmed by molecular genetic testing by the end of 2001, most of whom had been collected via research cohorts. However, there are clearly large numbers of patients who remained misdiagnosed due to unfamiliarity with monogenic diabetes because, if monogenic diabetes accounts for 1% of all diabetes, the minimum prevalence in the UK is reported to be approximately 6,000 cases or 108 per million.5 Identifying those with monogenic diabetes allows not only correct diagnosis and treatment, including cessation of insulin injections in most, but also genetic counselling about the risk to children, the likely progression of the condition and appropriate screening for family members.
Fig 1.
Timeline indicating significant developments. GDN = genetic diabetes nurse; MODY = maturity-onset diabetes of the young; RCT = randomised controlled trial; SU = sulfonylurea.
In response to this widespread lack of knowledge of monogenic diabetes, the authors developed the genetic diabetes nurse (GDN) project. This innovative project has been running since 2002 and is a national network of highly trained diabetes specialist nurses (DSNs) (Fig 2) who provide local expertise in monogenic diabetes across the UK, effectively and rapidly integrating new genetic knowledge into clinical care.11 This project was recognised as an exemplar in the Department of Health's White Paper, Our Inheritance, Our Future, for building genetics into mainstream services.12 The current paper evaluates 10 years of this national network, aims to assess whether this educational scheme resulted in increased diagnosis of monogenic diabetes and illustrates how this project could be used as a model for the effective translation of genetic knowledge into clinical care across a range of conditions. As this describes a service evaluation, ethical approval was not required.
Fig 2.
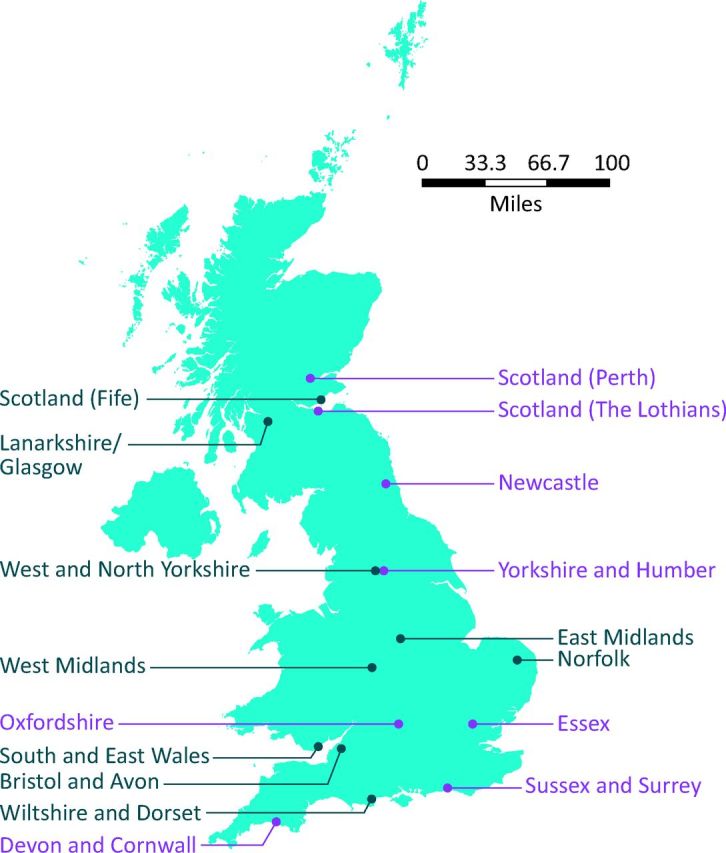
Location of genetic diabetes nurses and monogenic clinics. -Monogenic clinics are shown in purple. GDN = genetic diabetes nurse.
Establishing the genetic diabetes nurse service
Initial funding from the Department of Health to increase the integration of genetics into clinical care enabled the GDN project to be developed.13 The GDN posts were advertised through word of mouth via clinicians who had previously referred patients to the diagnostic genetic testing service and interested DSNs were identified. The nurses were seconded for one session (3.5 hours) per week to the project and trusts reimbursed their salary for these hours and associated travel costs. Each nurse was initially seconded to the role for a period of 12 months; this was continued subject to satisfactory progress and achievement. The GDNs remain embedded within their diabetes clinical teams as DSNs but are allocated a geographical region to cover as part of their role. Additional funding was later obtained from the charity Diabetes Foundation and the Scottish Executive, which enabled the project to increase the network of GDNs with ten intakes of new nurses over the next 10 years.
The role of the genetic diabetes nurses
The GDNs are trained as regional experts and increase knowledge and awareness of monogenic diabetes by disseminating information through presentations to healthcare professionals across the country. The GDNs aim to aid identification of patients who may have monogenic diabetes, increase numbers of referrals for genetic testing and ensure accurate diagnosis through positive molecular genetic test results. GDNs also guide management and treatment of patients with monogenic diabetes and provide ongoing support to families and clinicians.
Genetic diabetes nurse training
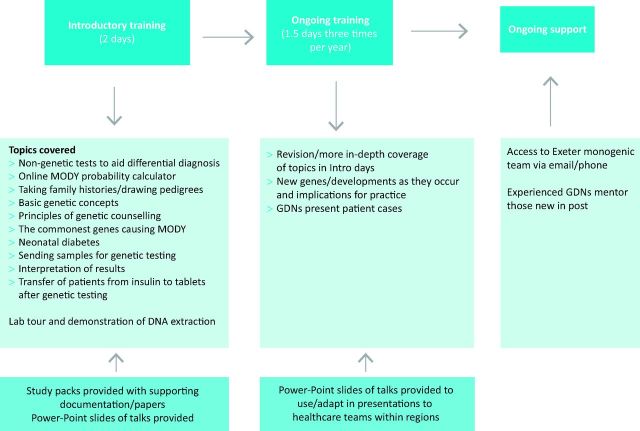
Introductory study days (2 days) cover basic topics relating to genetics and monogenic diabetes, and ongoing study days (1.5 days every 4 months) include in-depth sessions on these topics as well as updates as they occur (Fig 3). The sessions are given by the multidisciplinary Exeter team of doctors, scientists, nurses and genetic counsellors. PowerPoint slides from these sessions are provided for GDNs to use in presentations to healthcare teams within their regions.
Fig 3.
Introductory and ongoing training and -support of all genetic diabetes nurses, organised and delivered by a centre of excellence in Exeter. GDN = genetic diabetes nurse. MODY = maturity onset -diabetes of the young.
GDNs have email and phone access to the authors and Exeter colleagues for support and guidance about clinical cases. GDNs who are new in post are also allocated a more experienced GDN as a mentor; this person can provide additional assistance. Interim analysis indicated that the GDN posts enabled DSNs to extend their skills13 while increasing recognition and awareness of monogenic diabetes across the UK.11 Quality control is ensured through discussions and feedback with M Shepherd and following measures of activity, presentations and referrals.
Aims
The authors aim to evaluate whether training specialist nurses is an effective means of disseminating genetic information into clinical care.
Methods
The effectiveness of the GDNs was measured by evaluating: the numbers of patients whom they referred for genetic testing and the proportion of these receiving a positive genetic test result, and their presentations to healthcare professionals and the services/support that they offered to families with a confirmed diagnosis. GDNs were asked to complete feedback forms after each presentation given across their region, which provided a summary of the location, staff present and GDNs’ reflections on the session, any difficulties encountered and followup organised. The GDNs provided evaluation forms for the attendees to complete which scored the presentation on educational value, quality and usefulness of the session. Feedback and evaluation forms were then collated and reviewed by the Exeter team.
The numbers of patients referred by the GDNs for genetic testing were monitored and the appropriateness of referrals, proportion of positive test results and numbers of affected family members tested were evaluated. GDNs were encouraged to initiate specialist monogenic clinics within their regions and the location and frequency of these clinics were recorded. GDNs supported patients during genetic testing and subsequent treatment change and provided details to the Exeter team. Ongoing assessment of the cases that the GDNs discussed and monitoring of their monthly activity were recorded. Review meetings have been held individually with all GDNs, and any GDNs underperforming when measured against these criteria or struggling with the complexity of the role, despite additional support and guidance, have had their posts discontinued.
Results
To date 52 GDNs have been trained in ten intakes since 2002.
Referrals for genetic testing
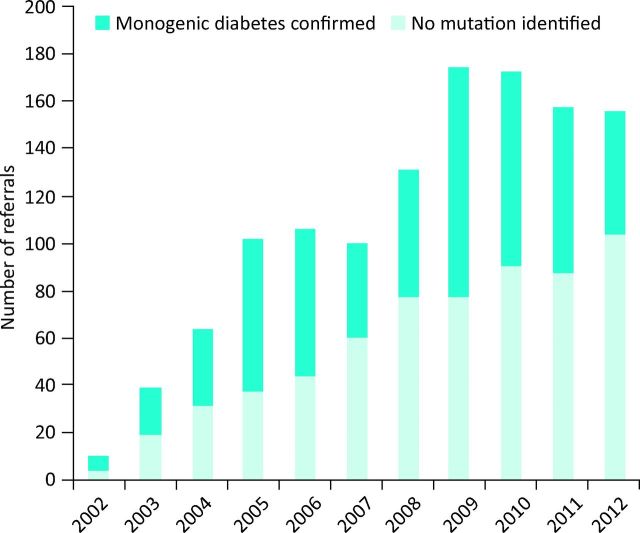
GDNs have helped increase yearly referral rates for genetic testing of diabetic patients (Fig 4) from a total of 111 in 2001 to 635 in 2012, and the total number of UK patients with a confirmed diagnosis of monogenic diabetes has also increased from 483 in 2001 to 2,092 in 2012. GDNs have a higher positive pick-up rate than patients referred from elsewhere (255/710 [36%] vs 661/2,935 [23%], p<0.0001), indicating that they are better at detecting patients most likely to have monogenic diabetes. GDNs have also increased referrals of family members for genetic testing compared with others (165/255 [65%] vs 345/661 [52%], p<0.0001), indicating an increased awareness of the importance of followup of other family members who may also have been incorrectly labelled as having type 1 or 2 diabetes.
Fig 4.
Total genetic diabetes nurse referrals for genetic testing by year with identification of positive cases.
Since being in post I have referred 164 patients for genetic testing; 81 of these now have a confirmed genetic diagnosis, which has allowed transfer to the most appropriate treatment and followup of family members.
(GDN, Bristol)
Dissemination of knowledge via presentations to healthcare professionals
GDNs have given 524 presentations to 6,304 healthcare professionals across the UK, raising awareness of monogenic diabetes. These talks have been highly evaluated, with 99% of the presentations rated by attendees as very good or excellent for both educational value and quality.
I have given 61 presentations throughout my region to 984 professionals who now refer possible patients to me.
(GDN, Devon and Cornwall)
GDNs have also worked collaboratively across regions to organise study days for staff across wider geographical areas.
Development of services
As a result of their training, the GDNs have created 9 UK-wide specialist monogenic clinics, reviewing >1,000 individuals from 710 families in collaboration with local diabetologists, improving the care offered for patients with monogenic diabetes within these regions. The GDNs and clinicians review patients and families with known or suspected monogenic diabetes within these clinics. Patients can be referred from elsewhere and the monogenic clinics are a forum for training other local staff.
In 2005 there was one MODY [maturity-onset diabetes of the young] patient in Brighton; we now have 55 confirmed MODY patients in the area, resulting in significant changes to treatment, including transfer of 11 patients from long-term insulin to sulfonylureas. Our monogenic clinic is held fortnightly and is well attended.
(GDN, Sussex)
Support for families
GDNs have supported more than 100 patients able to transfer from long-term insulin injections to sulfonylurea tablets after their genetic diagnosis. These patients were previously misdiagnosed as having type 1 diabetes and were inappropriately treated with unnecessary insulin injections. The role of the GDN was crucial in identifying these patients and ensuring a smooth transition from insulin injections to oral agents, leading to improvements not only in diagnosis and treatment, but also in the followup of other family members
Ongoing professional development of the genetic diabetes nurses
The GDN project has also provided opportunities for professional development. Five of the GDNs currently in post have gained further training at MSc level in genetic counselling, practical genetics and genetic science, which has enhanced their knowledge and credibility within the role. GDNs have presented posters and given talks at national and international conferences and published13–21 as a consequence of their role.
Discussion
The GDN project has been successful in increasing awareness of monogenic diabetes across the UK, leading to better care, more appropriate treatment/family followup, and considerable cost savings for the NHS for those patients able to transfer from insulin to sulfonylureas. The project has recruited and trained a team of highly motivated GDNs who have been able to share their expertise in monogenic diabetes with a wide range of healthcare professionals across the UK. The ongoing training, as well as regular contact with the Exeter monogenic diabetes team, enables rapid distribution of genetic information after new discoveries.
Although the GDN project costs are modest (<£100k/year), obtaining funding has been challenging because the GDNs work across regions and typical funding mechanisms are not transferable. National funding for very rare conditions, provided through specialist commissioning, was limited to diseases affecting fewer than 500 patients in the UK, and was therefore not applicable to monogenic diabetes. The Department of Health, the Diabetes Foundation and the Scottish Executive have all supported the project financially; however, continued sustainable funding remains problematic.
The GDN project may be seen as a model for the translation of genetics into clinical care in other areas because the rapid increase in genetic knowledge cannot possibly be managed by the current resources of clinical genetics teams. Specialist nurses work across a range of conditions in which specific genetic causes of disease are now recognised, eg in cancer and cardiac services, so they could be trained using a similar innovative approach of ongoing regular training, developed and coordinated by a centre of excellence. Developing the skills of specialist nurses using this model of training and dissemination could be a cost-effective and successful means of rapidly increasing genetic knowledge into clinical care across the NHS. (See www.diabetesgenes.org for further information about genetic testing and the GDN network.) n
Acknowledgements
With thanks to the Department of Health, Diabetes UK, the Scottish Executive and the Diabetes Foundation for funding this project. Thanks also to all the genetic diabetes nurses who have taken part in this project for their enthusiasm and hard work in increasing awareness of monogenic diabetes.
M Shepherd, S Ellard and AT Hattersley are supported by the NIHR Exeter Clinical Research Facility. S Ellard and AT Hattersley are both Wellcome Trust Senior Investigators.
References
- 1.Bennett CL, Burke SE, Burton H, Farndon PA. A toolkit for incorporating genetics into mainstream medical services: Learning from service development pilots in England. BMC Health Services Res 2010;10:125. 10.1186/1472-6963-10-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farndon P, Bennett C. Genetics education for health professionals: strategies and outcomes from a national initiative in the UK. J Gen Coun 2008;17:161–9. 10.1007/s10897-007-9144-x [DOI] [PubMed] [Google Scholar]
- 3.Hammersley S, Oram R, Shepherd M, et al. Prevalence of non-type 1 diabetes in paediatric diabetes. Diabetic Med 2013;30:24(A67). 10.1111/dme.12090_14 [DOI] [Google Scholar]
- 4.Hudson MM, Shepherd M, Oram RA, et al. A population-based approach to genetic testing defines the prevalence of maturity onset diabetes of the young (MODY) in patients with diabetes diagnosed under 30 years as 3%. Diabetic Med 2013;30:24(A68). 10.1111/dme.12090_14 [DOI] [Google Scholar]
- 5.Shields BM, Hicks S, Shepherd MH, et al. Maturity onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010;53:2504–8. 10.1007/s00125-010-1799-4 [DOI] [PubMed] [Google Scholar]
- 6.Shepherd M, Shields B, Ellard S, et al. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabetic Med 2009;26:437–41. 10.1111/j.1464-5491.2009.02690.x [DOI] [PubMed] [Google Scholar]
- 7.Pearson ER, Starkey B, Powell RJ, et al. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003;362:1275–81. 10.1016/S0140-6736(03)14571-0 [DOI] [PubMed] [Google Scholar]
- 8.Humphries S, Pedersen K. GPs have key role in detecting familial hypercholesterolaemia. Practitioner 2011;255:25–7, 3. [PubMed] [Google Scholar]
- 9.Burton H, Alberg C, Stewart A. Mainstreaming genetics: A comparative review of clinical services for inherited cardiovascular conditions in the UK. Public Health Genomics 2010;13:235–45. 10.1159/000279625 [DOI] [PubMed] [Google Scholar]
- 10.Hadfield SG, Horara S, Starr BJ, et al. Family tracing to identify patients with familial hypercholesterolaemia: the second audit of the Department of Health Familial Hypercholesterolaemia Cascade Testing Project. Ann Clin Biochem 2009;46(Pt 1):24–32. 10.1258/acb.2008.008094 [DOI] [PubMed] [Google Scholar]
- 11.Shepherd M, Hattersley AT, Ellard S. Integration of the MODY link nurse project: 20 month evaluation. J Diabetes Nurs 2005;9:47–52. [Google Scholar]
- 12.Department of Health Our Inheritance, Our Future: Realising the potential of genetics in the NHS. London: DH, 2003. [Google Scholar]
- 13.Dudding S, McMahon H, Shepherd M. MODY link nurses: pushing the boundaries of diabetes nursing. J Diabetes Nurs 2005;9:7–10. [Google Scholar]
- 14.Cropper J, Shepherd M. Reassessing people with diabetes aged under 25 years. In: Phillips A. (ed), Principles of Diabetes Care: Evidence based management of diabetes for health professionals. London: Quay Books, 2011:299–310. [Google Scholar]
- 15.Graja A, Young J, Shepherd M, et al. Importance of genetic testing and recognition of neonatal diabetes: A case report. J Diabetes Nurs 2011;15:149–51. [Google Scholar]
- 16.John H, Flanagan SE, Corral R, et al. Neonatal diabetes is more than just a paediatric problem: 57 years of diabetes from a Kir6.2 mutation. Pract Diabetes Int 2005;22:342–4. 10.1002/pdi.867 [DOI] [Google Scholar]
- 17.Morel K, Colclough K, Vaughan N, Shepherd M. Increased awareness of genetic diabetes leads to identification of >50 patients with monogenic diabetes from Brighton and Hove. J Diabetes Nurs 2013;7:250–4. [Google Scholar]
- 18.Shepherd M, Pearson ER, Houghton J, et al. No deterioration in glycaemic control in HNF1α MODY following transfer from long term insulin to sulphonylureas. Diabetes Care 2003;26:3191–2. 10.2337/diacare.26.11.3191-a [DOI] [PubMed] [Google Scholar]
- 19.Shepherd M, Cropper J, Flanagan SE, et al. A diagnosis of monogenic neonatal diabetes can improve treatment and glycaemic control. J Diabetes Nurs 2010;15:25–9. [Google Scholar]
- 20.Shepherd M, Miles S, Jones J, et al. Differential diagnosis: Identifying people with monogenic diabetes. J Diabetes Nurs 2010;14:342–7. [Google Scholar]
- 21.Jones JD, Shepherd M, Ellard S, et al. Macrosomia and neonatal hypoglycaemia can be seen in the offspring of fathers with diabetes. Diabetic Med 2006;23:A17. [Google Scholar]