Abstract
The objective of this study was to evaluate the 25-year outcome of patients with primary Sjögren's syndrome (pSS). One hundred and fifty-two patients diagnosed with pSS (American–European classification criteria) were retrospectively and descriptively analysed (1986–2011). Of all 152 patients, 55.9% were alive, 18.4% had died and 25.7% discontinued follow-up (mostly due to old age). Malignancy affected 28.3% and non-Hodgkin's lymphoma (NHL) affected 10.5%. The adjusted risk for development of NHL was an odds ratio (OR) of 10.5 (95% confidence interval [CI]: 3.05–36.42) in patients with vasculitis (p<0.001), and OR 3.4 (95% CI 1.05–11.2) in the presence of glandular complications (parotid swelling, lymphadenopathy) (p < 0.041). Seventy-five patients (49.3%) developed other autoimmune diseases (autoimmune thyroid disease [15.8%], pulmonary fibrosis [7.2%] and vasculitis [10.5%]). Although the course of pSS is relatively benign, over 25 years patients experience more clinical complications than previously described. In addition, vasculitis and glandular manifestations were significant predictors for NHL.
KEYWORDS : Sjögren’s syndrome, long-term follow-up, autoimmune diseases, non-Hodgkin’s lymphoma, neoplasia
Introduction
Sjögren's syndrome (SS) is a systemic autoimmune epithelitis.1 It affects approximately 0.5% of the general population,2,3 making it one of the most prevalent systemic autoimmune diseases, second only to rheumatoid arthritis.4 One of the most well-described risks to patients with SS is the development of non-Hodgkin's lymphoma (NHL), the lifetime probability of which has been reported to be between 5% and 15%,5 or 20-fold higher than in the general population.3 In primary Sjögren's syndrome (pSS), there are a number of other less well-established clinical complications that add to the burden of disease. These include other types of malignancies, other autoimmune diseases,6 vasculitis7 and haematological disorders.8,9 Sinister complications such as the malignancies are typically late events whereas other complications accumulate over time.10
The aim of this study was to analyse the demographic, serological, immunological and phenotypic characteristics of 152 patients followed up in the Centre for Rheumatology, University College Hospital, London, over a long-term follow-up.
Patients and methods
Patients
Between January 1986 and December 2011, 152 patients had been diagnosed with pSS at the authors’ unit. All patients met the revised American–European classification criteria (2002) for pSS.11 Patients with secondary SS were excluded. The authors retrospectively reviewed case notes, computer records and primary health-care databases on an audit basis. They analysed demographic (age at diagnosis, sex, race [white, African–Caribbean, Asian, other], years of follow-up and outcome [alive, dead, lost to follow-up]), serological (antinuclear antibody [ANA], rheumatoid factor, anti-Ro/SSA, anti-La/SSB, anti-ribonucleoprotein [RNP]), histological (minor salivary gland lip biopsy) and clinical features. Clinical features were divided arbitrarily into glandular manifestations (parotid swelling, lymphadenopathy) and extraglandular manifestations (non-erosive arthritis, Raynaud's phenomenon, central and peripheral nervous system [CNS and PNS] disease, and other autoimmune diseases). It is important to note that this distinction is arbitrary, and that other groups may have classified differently.
The autoimmune diseases considered included thyroid (-hypo- and hyperthyroidism, autoimmune thyroiditis), dermatological (cutaneous lupus, psoriasis, vitiligo), gastrointestinal (autoimmune hepatitis, primary biliary cirrhosis, coeliac disease, pernicious anaemia, ulcerative colitis), renal (renal tubular acidosis, glomerulonephritis) and pulmonary autoimmune diseases (fibrosis, interstitial disease), as well as vasculitis, systemic lupus erythematosus, scleroderma, myositis, sarcoidosis, anti-phospholipid syndrome, rheumatoid arthritis, uveitis, polymyalgia rheumatica and autoimmune cytopenias. All aforementioned autoimmune diseases were diagnosed at least 1 year after pSS was diagnosed. Major haematological disorders were also noted, including -hyper- and hypogammaglobulinaemia, monoclonal gammopathy of unknown significance (MGUS) and autoimmune cytopenias. Minor haematological alterations such as anaemia or non-autoimmune cytopenias were not noted. Finally, we considered the onset of solid and haematological malignancies, with particular attention to NHL.
Statistical analysis
Epidemiological, serological and clinical features were descriptively analysed. Frequencies and percentages were used for categorical variables, and mean (standard deviation [SD]) or median (interquartile range [IQR]) was used for continuous variables. A univariant analysis (χ2 and Fisher's tests and Fisher's tests) was used to compare the four most prevalent and/or relevant clinical findings, which were as follows:
haematological disorders
all malignancies (including NHL)
NHL
other autoimmune diseases.
A multivariant analysis was used for statistically significant features. Logistic regression and bivariant analysis was also performed to avoid the presence of confounding variables and calculate the adjusted OR (odds ratio) and 95% confidence interval (95% CI). A two-tailed value of p<0.05 indicates statistical significance. The statistical analysis was performed using the IBM SPSS Statistic 19.0 (SPSS, Chicago, IL, USA).
Results
General characteristics
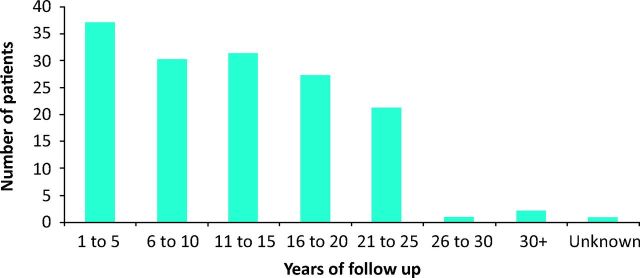
Our cohort was predominately white European (86.8%) and female (91.4%). The mean age of diagnosis was 54.4 years (SD = 13.5 years). The number of years of follow-up since a diagnosis was made ranged from 1 year to 47 years (median = 11; IQR 6–19) and are summarised in Fig 1; 39 patients (25.6%) discontinued follow-up at some point during the study period, mainly due to being too elderly to travel to the clinic easily. For these patients, the authors included clinical complications until the final follow-up appointment. Of the remaining 113 patients, 28 (18.5%) died at some point during the study period. The mean age of death was 72.1 years (SD = 12.3 years).
Fig 1.
Summary of years of follow-up for the study
Serologically, the presence of ANAs was the most frequent finding (75.7%) followed by anti-Ro/SSA antibodies and rheumatoid factor in just under 55%. Extraglandular manifestations were 3.4-fold more common than glandular manifestations (108 [71.1%] vs 31 [20.4%]). Of glandular manifestations, parotid swelling was the most common (n = 21; 13.8%), and of extraglandular manifestations, additional autoimmune diseases were the most common (n = 75; 49.3%).
Additional autoimmune diseases
Additional autoimmune diseases were the most common extraglandular manifestation in our cohort, with a total of 75 patients (49.3%) developing at least one (see Table 1 for autoimmune diseases included). Autoimmune thyroid disease was the most frequent of these (n = 24, 15.8%; 32.0% of autoimmune diseases), followed by vasculitis, dermatological and gastrointestinal autoimmune diseases, which occurred in 16 patients each (21.3% of global autoimmune diseases each). Of dermatological autoimmune diseases, cutaneous lupus was the most common (n = 4; 2.6%) and of gastrointestinal diseases, primary biliary cirrhosis was the most frequent (n = 6; 3.9%). Autoimmune lung and renal diseases were present in 11 (7.2%) and 10 (6.5%) patients, respectively. Twenty-nine patients (38.7%) developed more than one additional autoimmune disease, up to a maximum of four in one patient (0.7%). Table 2 compares those with and those without additional autoimmune diseases; a significantly higher incidence was found in females (72 [96.0%] vs 67 [87.0%]; p<0.01), those with positive anti-Ro/SSA (48 [64.0%] vs 37 [48.1%]; p<0.05), positive anti-La/SSB (33 [44.0%] vs 21 [27.3%]; p<0.03) and those who also had haematological disorders (46 [61.3%] vs 35 [45.5%]; p<0.05). There was no significant difference in years of follow-up in those who developed additional autoimmune diseases. Multivariant analysis was unable to demonstrate risk factors for the development of additional autoimmune diseases.
Table 1.
General characteristics of the cohort studied (n=152).
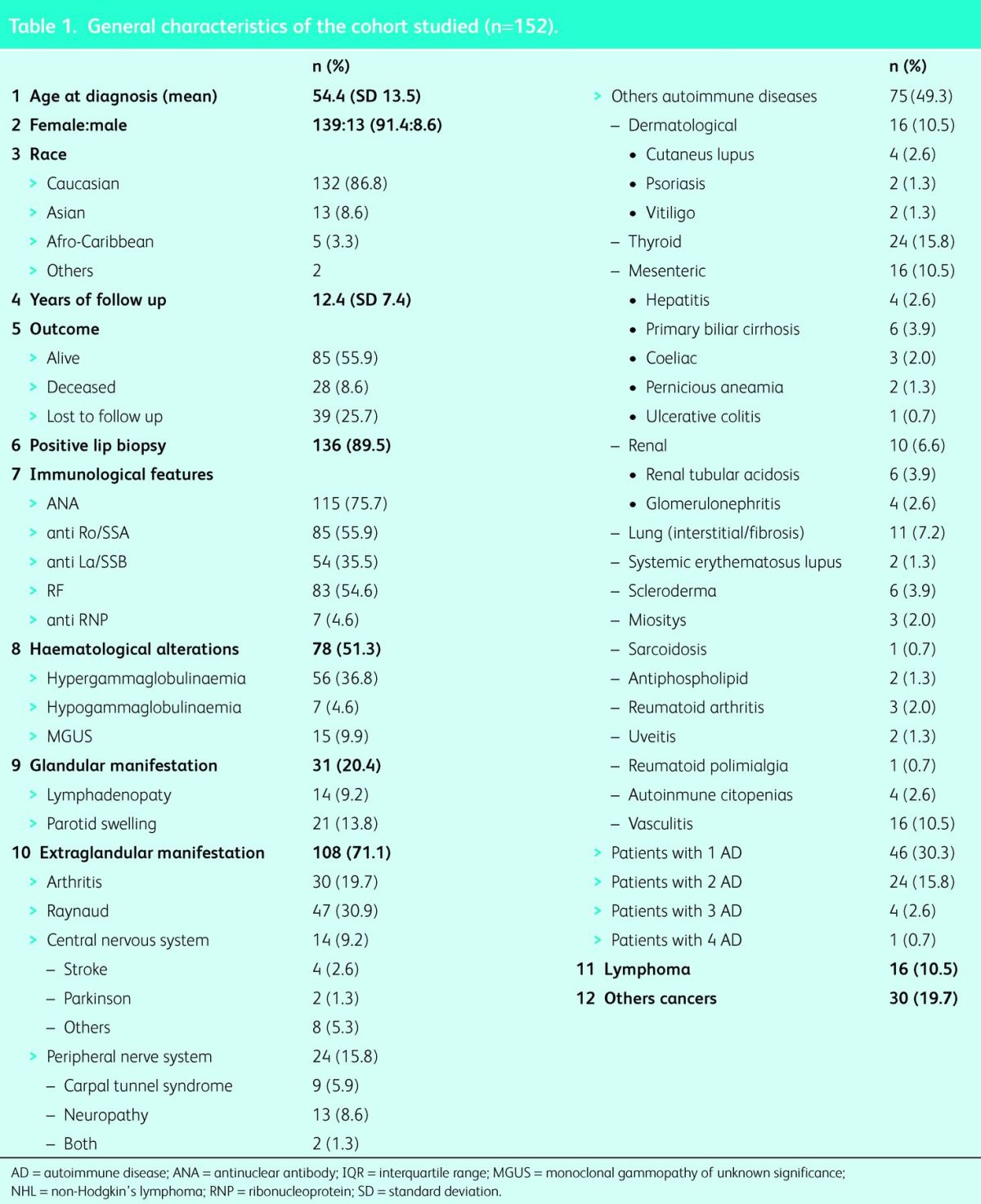
Table 2.
Primary Sjögren's syndrome with and without autoimmune diseases
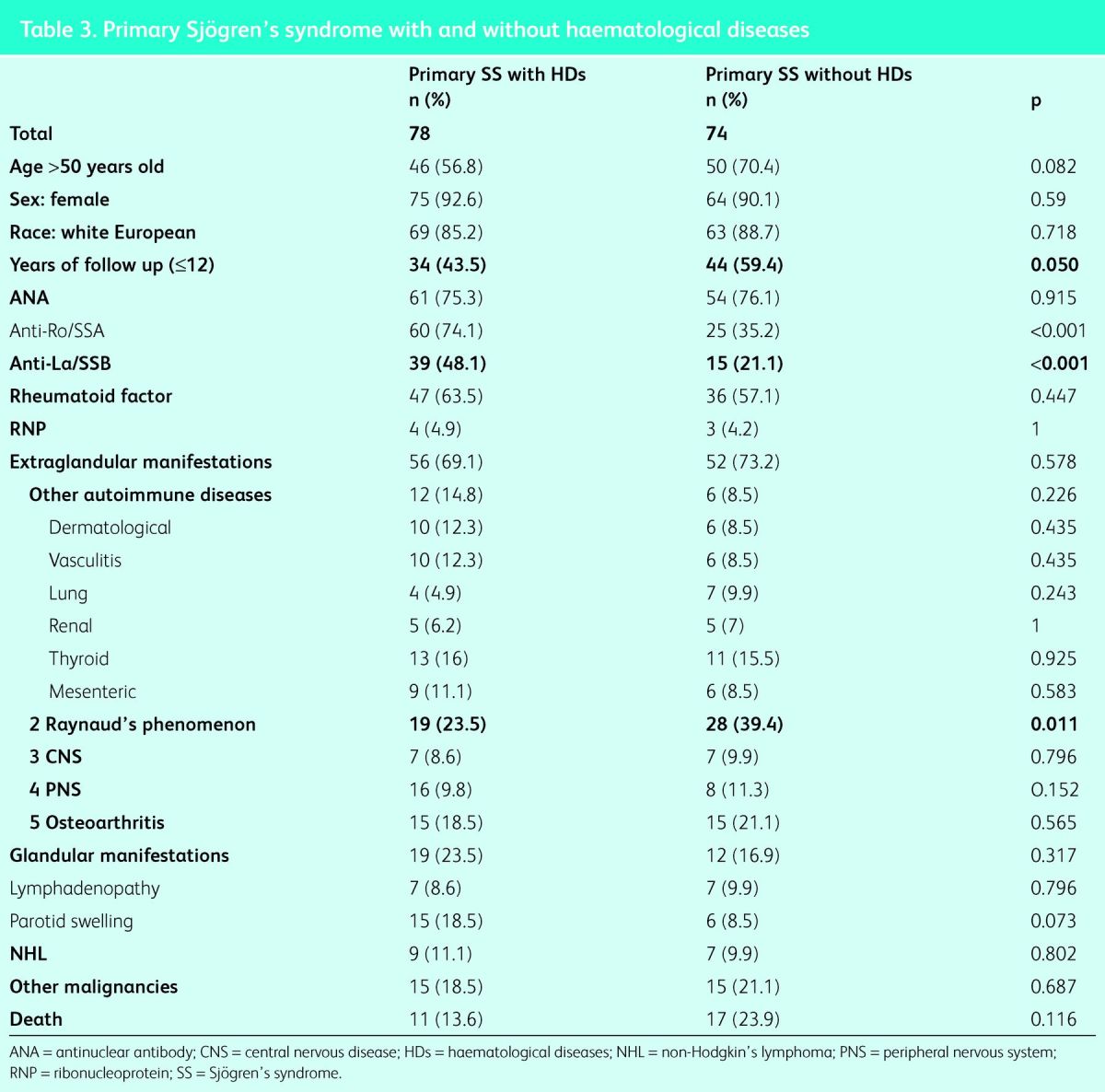
Haematological disorders
Seventy-eight patients (51.3%) developed haematological derangements. Under the arbitrary classification outlined in the methods, hypergammaglobulinaemia was the most common (n = 56; 36.8%), followed by MGUS (n = 15; 9.9%) and hypogammaglobulinaemia (n = 7; 4.6%). Table 3 compares those with and those without haematological disorders; a significantly higher incidence was found in patients who had a longer follow-up (312 years), and those with positive anti-Ro/SSA (60 [77.0%] vs 25 [33.8%]; p<0.001) and positive anti-La/SSB (39 [50.0%] vs 15 [21.1%]; p<0.001). Significantly fewer patients had Raynaud's phenomenon (10 [24.4%] vs 28 [37.8%]; p<0.011). The adjusted risk of developing haematological disorders was and OR of 0.3 (95% CI 0.1–0.8 in patients with Raynaud's phenomenon (p = 0.01), OR 1.1 (95% CI 1.01–1.2) in patients with a longer follow-up (p = 0.001), OR 0.7 (95% CI 0.2–1.9) in patients with positive anti-La/SSB (p = 0.478), and OR 0.2 (95% CI 0.1–0.5) in those with positive anti-Ro/SSA (p = 0.001).
Table 3.
Primary Sjögren's syndrome with and without haematological diseases
Malignancy
A total of 43 (28.3%) patients developed malignancy; 13 developed NHL alone, 3 developed NHL and a second cancer (melanoma in 2 cases, ovarian cancer in 1), and 27 developed haematological and solid malignancies besides NHL. This brought the total incidence of non-lymphoma cancers to 19.7% (n = 30) and total incidence of NHL to 10.5% (n = 16).
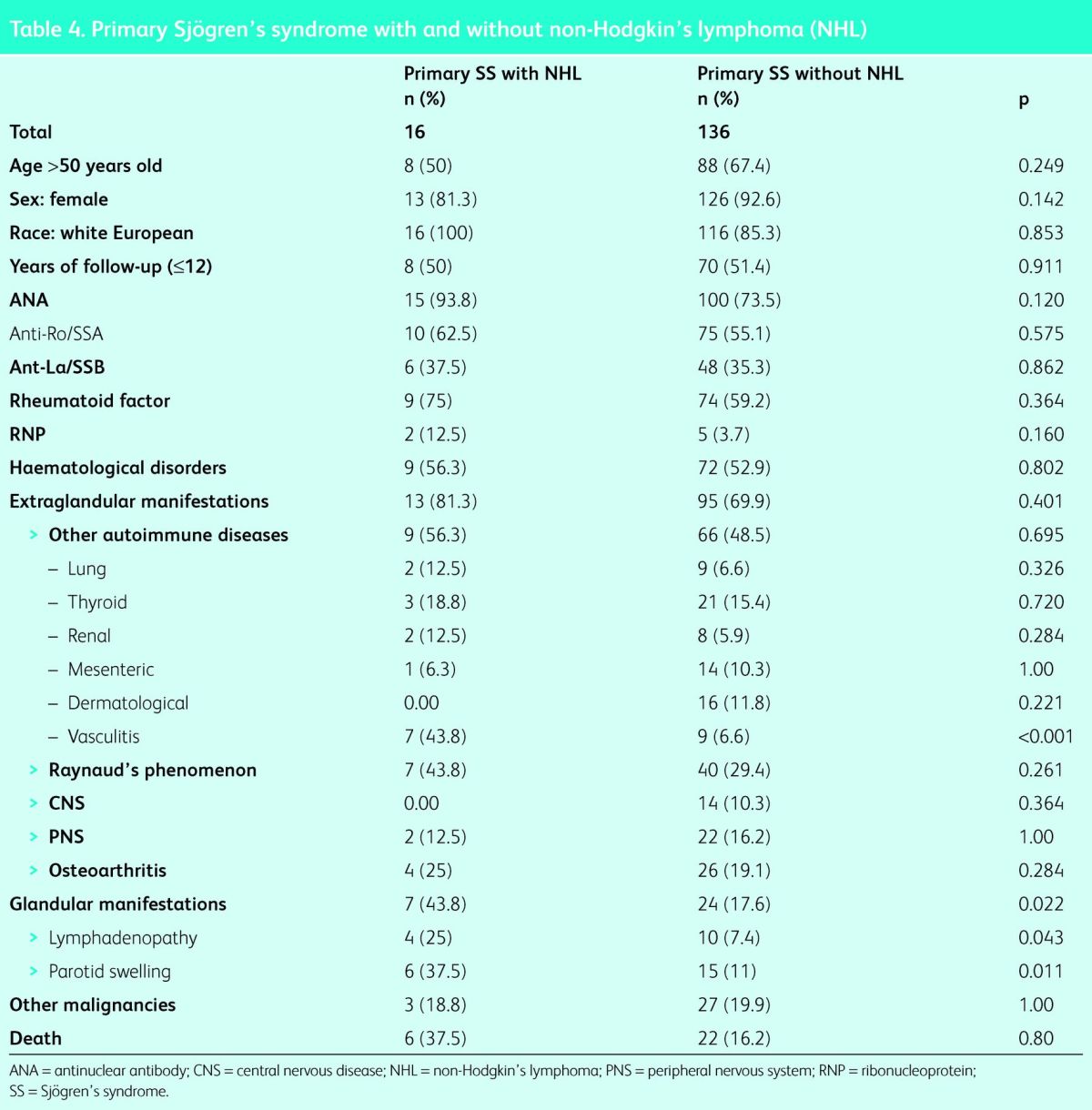
Non-Hodgkin's lymphoma
Patients diagnosed with NHL were compared with all other patients (Table 4). The parameters of sex, age, race, duration of disease, and haematological and immunological characteristics did not show any statistically significant differences. However, the incidence of glandular manifestations was significantly higher in the lymphoma group (7 [43.8%] vs 24 [17.6%]; p<0.05), as was the incidence of vasculitis (7 [43.8%] vs 7 [6.6%]; p<0.001). The incidence of other cancers and mortality were not significantly different between the two groups. In the multivariant analysis the adjusted risk for development of NHL was OR of 10.5 (95% CI 3.05–36.42) in patients with vasculitis (p<0.001), and OR 3.4 (95% CI 1.05–11.2) in the presence of glandular manifestations (p = 0.041).
Table 4.
Primary Sjögren's syndrome with and without non-Hodgkin's lymphoma (NHL)
All malignancies
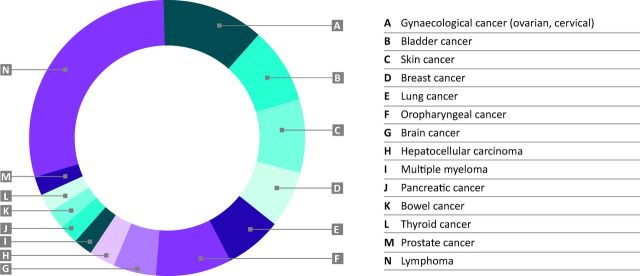
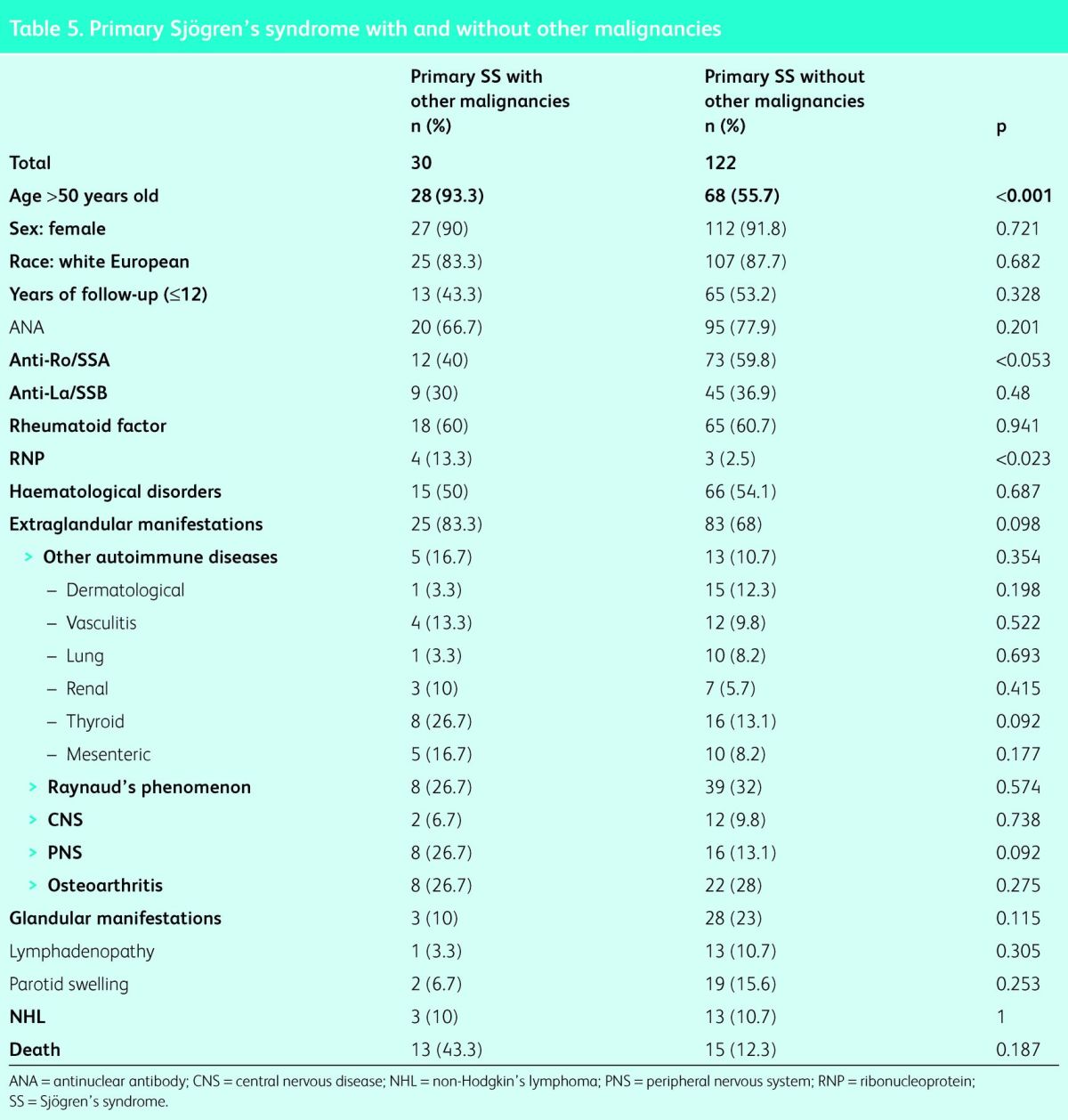
Other malignancies that the patients developed included: gynaecological (n = 5: 3 ovarian, 2 cervical), bladder cancer (n = 4), skin (n = 4: 3 melanomas, 1 basal cell carcinoma), lung (n = 3, including 1 mesothelioma), breast (n = 3), oropharyngeal (n = 3), CNS (n = 2: 1 cavernoma, 1 meningioma), and single cases of each of the following: hepatocarcinoma, multiple myeloma, pancreatic cancer, bowel cancer, thyroid cancer and prostate cancer. These are summarised in Fig 2. Table 5 compares the features of all patients who developed solid and haematological malignancies versus those who did not. The malignancy group was significantly older (>50 years: 28 [93.3%] vs 68 [55.7%]; p<0.001), significantly fewer were anti-Ro/SSA positive (12 [40.0%] vs 73 [59.8%]; p<0.05] and significantly more were RNP positive (4 [13.3%] vs 3 [2.5%]; p<0.02). There was no statistically significant difference in any other clinical feature. In the multivariant analysis the adjusted risk for the development of malignancies was OR of 9.6 (95% CI 2.1–42.9) in patients aged >50 years (p = 0.003), OR 0.6 (95% CI 0.3–1.4) for anti-Ro/SSA-positive patients (p = 0.609) and OR 4.9 (95% CI 0.9–25.6) in RNP-positive patients (p = 0.06).
Fig 2.
Summary of all the malignancies that the patients developed.
Table 5.
Primary Sjögren's syndrome with and without other malignancies
Discussion
Primary Sjögren's syndrome is not generally thought to be associated with life-expectancy-shortening complications, especially when it is compared with systemic lupus erythematosus and rheumatoid arthritis.12 However, trends in the development of more ominous complications of pSS appear to be latent.10 Despite new studies of outcome in pSS with large sample sizes, classification is hotly debated and few studies boast an average follow-up of >10 years.7,10,12–16 The current report demonstrates a retrospective analysis of 152 patients with pSS followed carefully over 25 years, with an average duration of follow-up of 12.5 years (SD = 7.4 years). This is the largest and longest study of pSS patients from the UK.10 The demographic and serological features of our population were similar to those previously described in the literature.10,14,16–21
The development of additional autoimmune diseases was found in just under half of the cohort (49.3%), which is more than others report.22 The most common of these was autoimmune thyroid disease, as demonstrated by previous studies.6,18,23,24 Importantly, 19.0% of patients developed more than one additional autoimmune disease, up to a maximum of four in one patient. This is consistent with the phenomenon of ‘polyautoimmunity’ as previously described.6 Relevant characteristics (p<0.05) of those who develop additional autoimmune diseases in our cohort were: female anti-Ro/SSa and anti-La/SSb positive, and haematological disorders. These results are supported by previous findings.10
The total malignancy rate in our cohort was higher than in any other study that the authors could identify,5,25 with over a quarter (28.3%) developing cancer, mostly due to an increased incidence of lymphoma. The relative prevalence of solid and haematological malignancies besides NHL was significantly related to older age (p<0.001). These observations argue against pSS being a direct risk factor for the development of systemic malignant transformation. Evidence for such a relationship is much firmer in the case of NHL. However, with only two other studies to compare,5,25 there is a lack of data to corroborate this finding due to a shortage of extended follow-up studies. Nevertheless, it has long been known that systemic autoimmune diseases can potentiate dysplastic changes. This in turn is related to the aforementioned breakdown of immune tolerance, supported by the fact that RNP-positive patients had a 4.9-fold increased adjusted risk of any kind of malignancy (95% CI 0.9–25.6; p<0.06) in the multivariant analysis, and those who developed non-lymphoma malignancies were less likely to be anti-Ro/SSA positive (p<0.053) in the univariant analysis.
NHL is the only malignancy with a proven link to pSS.5 Occurring in 10.5% of patients, NHL was the single most common subtype of malignancy in this cohort. This is higher than national statistics22 and previous data,7,12,13,15,18,25–27 but similar to the rates found by Kruize et al14 (10.0%) and others. This is probably due to chronic local inflammation28 coupled with intrinsic abnormalities in B cells and B-cell activating factors,1 as explained in depth by Youinou et al.28,29
There are wide ranges of suggested predictors for the development of NHL in pSS. However, in the authors’ cohort only patients with vasculitis and glandular manifestations demonstrated a 10.5-fold and 3.42-fold increased adjusted risk for developing NHL, respectively (vasculitis: 95% CI 3.05–36.42; p<0.001; glandular manifestations: 95% CI 1.05–11.2; p = 0.041). As supported by Sutcliffe et al,27 no serological mediators are implicated as risk factors. Serological markers that were not tested should be analysed prospectively in the future to confirm this. It was surprising that there was no relationship between NHL and the duration of follow up, as previously shown.15 This is possibly due to the technical limitations of this retrospective study and the need for a higher sample size.
Importantly, this study found that 18.8% of patients with NHL had a second cancer. This is supported by a previous study.27 Here it is possible that defective DNA-repair mechanisms and cytotoxic therapies contribute. However, the latter are unlikely because very few of the patients in this study received cytotoxic therapy, but they could form the basis of further study. Although NHL does not significantly decrease life expectancy,13 this second malignancy is often a source of mortality.
Haematological disorders affected more than 50% of the cohort. Hypergammaglobulinaemia (36.8%) and MGUS (8.6%) were most common. These findings were significantly related to years of follow-up and, although they were more common in those with positive anti-Ro/SSA (p<0.001) and positive anti-La/SSb (p<0.001), this could not be confirmed by the multivariant analysis. These findings implicate both a temporal and an immunological relationship for haematological disorders in pSS. Interestingly, haematological disorders were inversely correlated to those with Raynaud's phenomenon (p<0.01; OR <1). However, the incidence of Raynaud's phenomenon is difficult to interpret. A recent study has suggested that MGUS is associated with a poor prognosis in pSS.9 However, this study does not show significant differences in glandular manifestations, lymphoma, other cancers, other extraglandular manifestations or mortality in the haematological disorders group.
Other complications in this study's cohort were common and variable: almost half the patients displayed Raynaud's phenomenon; and non-erosive arthritis rates were high, related to the high mean age of the cohort. Just over a quarter of patients developed CNS and PNS disturbances, reflecting most of the previous studies.10,20 However, neuropsychiatric complications should be interpreted with caution because they are difficult to document reliably and extremely heterogeneous in the literature. The most common nervous manifestation in this cohort was peripheral neuropathy.
To conclude, the present study summarises a single centre's 25-year experience of 152 patients with pSS, followed for a mean of 12.4 years. Globally, these data demonstrate that patients with pSS experience an increased rate of malignancy (mainly NHL), and additional autoimmune and haematological disorders. The findings indicate that shorter outcome studies are at risk of underestimating the burden of pSS and a universal thread has been the importance of a holistic and vigilant approach to follow up.
References
- 1.Tzioufas AG, Voulgarelis M. Update on Sjögren's syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Practice Res Clin Rheumatol 2007;21:989–1010. 10.1016/j.berh.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 2.Zenone T. Parotid gland non-Hodgkin lymphoma in primary Sjögren syndrome. Rheumatol Int 2011;32:1387–90. 10.1007/s00296-011-1851-9 [DOI] [PubMed] [Google Scholar]
- 3.Jonsson MV, Theander E, Jonsson R. Predictors for the development of non-Hodgkin lymphoma in primary Sjögren's syndrome. La Presse Médicale 2012;41:e511–16. 10.1016/j.lpm.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 4.Youinou P, Pers J-O. Latest update on the primary Sjögren's syndrome. La Presse Médicale 2012;41:e437–9. 10.1016/j.lpm.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Feng S, Yan S, et al. Incidence of malignancy in primary Sjögren's syndrome in a Chinese cohort. Rheumatology 2009;49:571–7. 10.1093/rheumatology/kep404 [DOI] [PubMed] [Google Scholar]
- 6.Lazarus MN, Isenberg DA. Development of additional autoimmune diseases in a population of patients with primary Sjögren's -syndrome. Ann Rheum Dis 2005;64:1062–4. 10.1136/ard.2004.029066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson B, Kelly C, Griffinths I. Primary Sjögren's syndrome in the North East of England: a long-term follow-up study. Rheumatology 1999;38:245–53. 10.1093/rheumatology/38.3.245 [DOI] [PubMed] [Google Scholar]
- 8.Khattri S, Barland P. Primary Sjögren's syndrome and autoimmune cytopenias: a relation often overlooked. Bull NYU Hosp Joint Dis 2012;70:130–2. [PubMed] [Google Scholar]
- 9.Brito-Zerón P, Retamozo S, Gandía M, et al. Monoclonal gammopathy related to Sjögren syndrome: A key marker of disease prognosis and outcomes. J Autoimmun 2012;39:229–33. 10.1016/j.jaut.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 10.Ramos-Casals M, Solans R, Rosas J, et al. Primary Sjögren dyndrome in Spain. Medicine 2008;87:210–19. 10.1097/MD.0b013e318181e6af [DOI] [PubMed] [Google Scholar]
- 11.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krylova L, Isenberg D. Assessment of patients with primary Sjögren's syndrome – outcome over 10 years using the Sjögren's Syndrome Damage Index. Rheumatology 2010;49:1559–62. 10.1093/rheumatology/keq086 [DOI] [PubMed] [Google Scholar]
- 13.Pariente D, Anaya J-M, Combe B, et al. Non-Hodgkin's lymphoma associated with primary Sjogren's syndrome. Eur J Med 1992;1:337–42. [PubMed] [Google Scholar]
- 14.Kruize AA, Hene R, van der Heide A, et al. Long-term followup of patients with Sjögren's syndrome. Arthritis Rheum 1996;39:297–303. 10.1002/art.1780390219 [DOI] [PubMed] [Google Scholar]
- 15.Solans-Laqué R, López-Hernandez A, Angel Bosch-Gil J, et al. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren's syndrome. Semin Arthritis Rheum 2011;41:415–23. 10.1016/j.semarthrit.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 16.Theander E, Manthorpe R, Jacobsson LTH. Mortality and causes of death in primary Sjögren's syndrome: A prospective cohort study. Arthritis Rheum 2004;50:1262–9. 10.1002/art.20176 [DOI] [PubMed] [Google Scholar]
- 17.Skopouli F, Dafni U, Ioannidis J, Moutsopoulos H. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum 2000;29:296–304. 10.1016/S0049-0172(00)80016-5 [DOI] [PubMed] [Google Scholar]
- 18.Malladi AS, Sack KE, Shiboski S. et al. Primary Sjögren's syndrome as a systemic disease: A study of participants enrolled in an -international Sjögren's syndrome registry. Arthritis Care Res 2012:64:911–18. 10.1002/acr.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botsios C, Furlan A, Ostuni P. et al. Elderly onset of primary Sjögren's syndrome: Clinical manifestations, serological features and oral/ocular diagnostic tests. Comparison with adult and young onset of the disease in a cohort of 336 Italian patients. Joint Bone Spine 2011;78:171–4. 10.1016/j.jbspin.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Carrasco M, Ramos-Casals M, Rosas J, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore) 2002;81:270–80. 10.1097/00005792-200207000-00003 [DOI] [PubMed] [Google Scholar]
- 21.Bournia V-K, Vlachoyiannopoulos PG. Subgroups of Sjögren syndrome patients according to serological profiles. J Autoimmun 2012;39:15–26. 10.1016/j.jaut.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 22.Office of National Statistics Cancer Statistics Registrations, England. London: ONS, 2012. [Google Scholar]
- 23.d’Arbonneau F, Ansart S, Berre RL, et al. Thyroid dysfunction in primary Sjögren's syndrome: A long-term followup study. Arthritis Rheum 2003;49:804–9. 10.1002/art.11460 [DOI] [PubMed] [Google Scholar]
- 24.Amador-Patarroyo MJ, Arbelaez JG, Mantilla RD, et al. Sjögren's syndrome at the crossroad of polyautoimmunity. J Autoimmun 2012;39:199–205. 10.1016/j.jaut.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 25.Wouters EJ, van Leeuwen N, Bossema ER, et al. Physical activity and physical activity cognitions are potential factors maintaining fatigue in patients with primary Sjögren's syndrome. Ann Rheum Dis 2011;71:668–73. 10.1136/ard.2011.154245 [DOI] [PubMed] [Google Scholar]
- 26.Garcia Portales R, Belmonte Lope M, Camps Garcia M, et al. Immunogenetics of the Sjogren's syndrome in southern Spain. An Med Intern 1994;11:56–61. [PubMed] [Google Scholar]
- 27.Sutcliffe N, Inanc M, Speight P, Isenberg DA. Predictors of -lymphoma development in primary Sjögren's syndrome. Semin Arthritis Rheum 1998;28:80–7. 10.1016/S0049-0172(98)80040-1 [DOI] [PubMed] [Google Scholar]
- 28.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of -lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med 2005;165:2337–44. 10.1001/archinte.165.20.2337 [DOI] [PubMed] [Google Scholar]
- 29.Youinou P, Devauchelle-Pensec V, Pers JO. Significance of B cells and B cell clonality in Sjögren's syndrome. Arthritis Rheum 2010;62:2605–10. 10.1002/art.27564 [DOI] [PubMed] [Google Scholar]