Key points
Sarcopenia is a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength, with a risk of adverse outcomes
Pathophysiology of sarcopenia is complex, involving muscle, neural and hormonal changes
Differential diagnosis has to be carried out with starvation and cachexia
A comprehensive multidimensional approach to investigate the aetiology of sarcopenia as a geriatric syndrome is recommended
Nutrition and physical exercise are the cornerstones of intervention in sarcopenia
Introduction
The concept of sarcopenia is encountered with increasing frequency in practice and research, not only in geriatric medicine but also in a range of other specialties. Although sarcopenia is common and has huge personal and societal costs, there is still no broadly accepted definition, no code in the international and statistical classification of diseases and health-related problems, tenth edition (ICD-10), and no treatment guideline.
The word sarcopenia derives from the Greek roots sarx, for flesh, and penia, for loss. It was first used in 1988 at a meeting convened in Albuquerque, USA, to discuss assessment of health and nutrition in older populations and refers to the age-related decline in muscle mass and function that affects ambulation, mobility, nutrient intake and status, and functional independence.1
Sarcopenia can be viewed as an organ failure (muscle insufficiency) and is usually chronic but can develop acutely (for example, during hospital admission). It is linked, through physical frailty, to the development of physical disability.
Pathophysiology
The pathophysiology of sarcopenia is complex, involving muscle and associated neural and hormonal regulation.2 With normal ageing, the quality of muscle fibres slowly deteriorates3 and peak power, shortening speed and elasticity decline slowly. The weakness of the muscle fibres can be explained by the interaction of several age-related changes, including loss of anabolic stimuli due to decline in the concentration of testosterone and other anabolic hormones and age-associated subclinical inflammation. This latter aspect may be partially reversed with exercise training.
Reductions in the number and activation of satellite cells also occur in older people, especially those associated with type IIA fibres, which may reduce the regenerative capacity of muscle fibres and compensatory capacity. Myostatin levels also increase with age. As myostatin is a negative regulator of muscle mass, an increase in circulating levels may lead to muscle atrophy. Changes in regulation of the myostatin gene may also contribute to age-related changes in the protein profile of muscle.
Neurological control of movement has only very recently received attention as a direct contributor to sarcopenia at different levels, especially the loss of the motor end plate.4 Metabolic and endocrine age-related changes are also involved in the genesis of sarcopenia.5
Definition
When the word sarcopenia was first used, most experts acknowledged that it referred to a loss of muscle mass, function and quality. However, most epidemiological studies on the prevalence and characteristics of sarcopenia only used definitions based on muscle mass due to the lack of clear agreement on what parameters should be used to measure muscle function. Magnetic resonance imaging and computerised tomography were used in experimental settings and dual-energy X-ray absorptiometry (DXA) and bioimpedance analysis (BIA) in epidemiological studies. However, muscle mass has not proved to be a good predictor of relevant clinical outcomes (mortality and physical disability). Moreover, both DXA and BIA have important limitations in measuring muscle mass, with substantial variability depending on the equation used to estimate lean body mass.6,7
Although the definition of sarcopenia based on loss of muscle mass alone has served the scientific community fairly well, it has been less satisfying for clinicians, the pharmaceutical industry and regulatory agencies. Unlike measurements of bone mineral density, measurement of muscle mass has not been adopted widely by clinicians. Some organisations have therefore put efforts into developing new definitions that included something more than muscle mass.
In 2011, the Society for Sarcopenia, Cachexia and Wasting Disorders proposed the term ‘sarcopenia with limited mobility’ to define people with a need for therapeutic intervention.8 Sarcopenia with limited mobility is defined as loss of muscle mass (lean appendicular mass corrected for height squared of more than two standard deviations below that of healthy people aged 20–30 years of the same ethnic group) and low walking speed (≤1 m/s during a 4-metre walking test or less than 400 m during a 6-minute walking test).
The European Working Group on Sarcopenia in Older People (EWGSOP) – an expert group convened in 2010 by the European Union Geriatric Medicine Society, the European Society of Clinical Nutrition and Metabolism, and other partners – went a step further by including loss of muscle function in the term sarcopenia. This group defines sarcopenia as a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength, with a risk of adverse outcomes such as physical disability, poor quality of life and death.9 The operational definition recommends using the presence of both low muscle mass and low muscle function (strength or performance) to diagnose sarcopenia. Diagnosis thus requires documentation of criterion 1 plus documentation of either criterion 2 or criterion 3 (Box 1). This definition has been shown to be useful for community and nursing home populations10,11 and is strongly linked with adverse outcomes (for example, mortality and falls).12,13
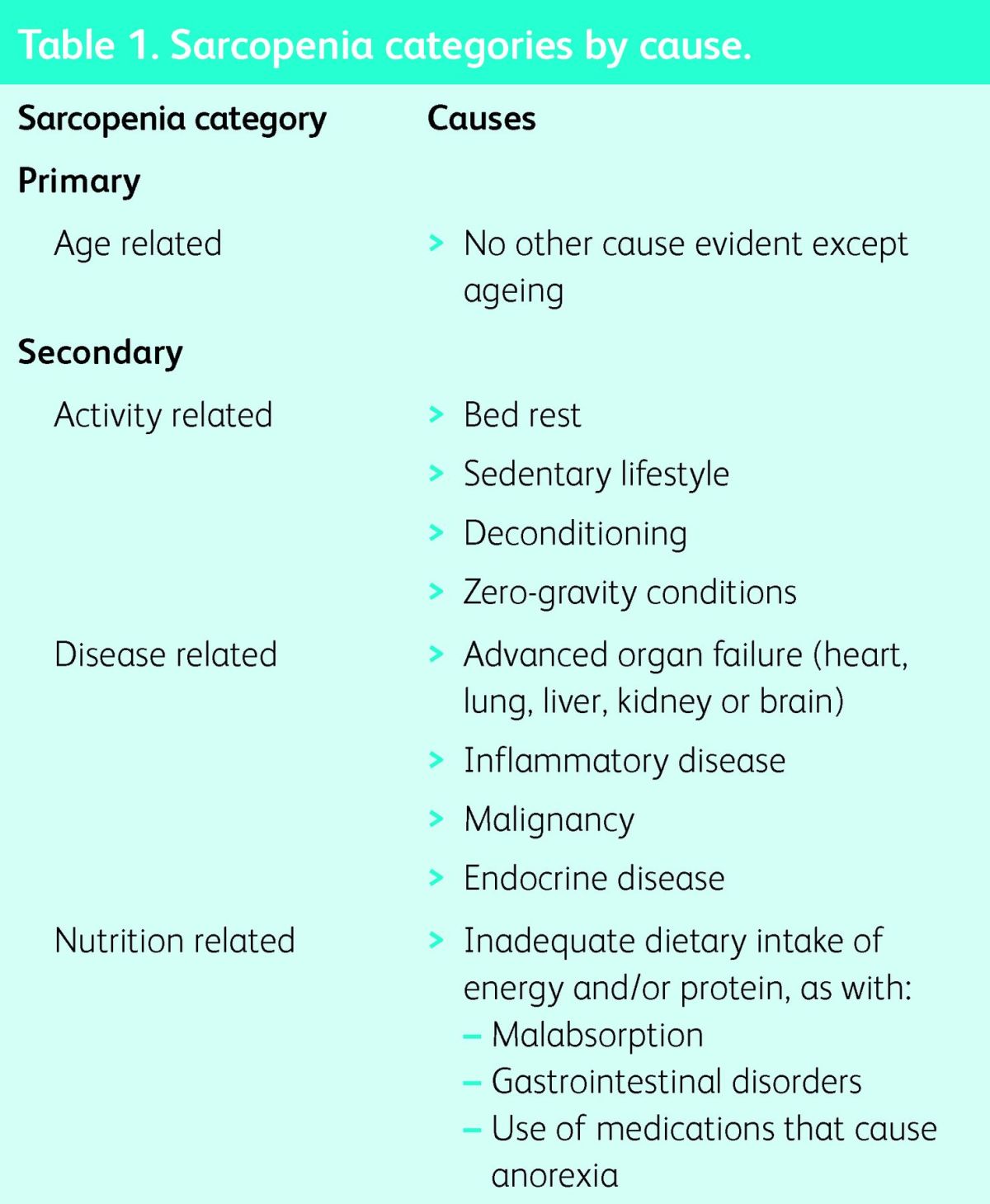
The EWGSOP also divides sarcopenia into categories and proposes the terms primary and secondary sarcopenia. Sarcopenia can be considered primary (or age related) when no other cause is evident apart from advanced age, while sarcopenia can be considered secondary when one or more other causes are evident (Table 1). In many older people, the aetiology of sarcopenia is multifactorial, so it may not be possible to characterise each individual as having exclusively primary or secondary sarcopenia.
Table 1.
Sarcopenia categories by cause.
The EWGSOP definition is now used widely and research is needed to determine whether its use in clinical practice can help prevent disability and other outcomes. However, there are still many active topics for debate, including measurement of muscle mass in clinical practice, cut-off values for parameters used in definitions, measurement of muscle quality, the role of fat and sarcopenic obesity, approaches to case finding, and the most relevant outcomes for therapeutic or preventive intervention.14
Detection of sarcopenia in clinical practice
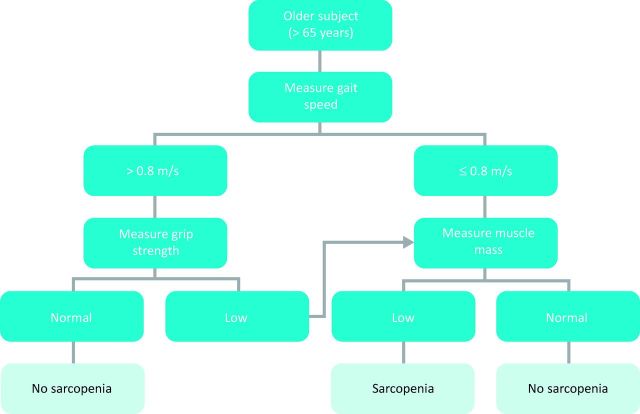
Identifying subjects with sarcopenia in clinical practice is an important task in the quest to prevent age-associated disability. Case finding is directly linked with the definition used and may take place in the general population or in high-risk groups. The EWGSOP proposes a population approach, recommending screening of all people aged over 65 years. However, it proposes a gradualist approach, starting with measurement of gait speed using a cut-off value of <0.8 m/s to identify those at risk of sarcopenia. People so identified should undergo further testing to determine whether they achieve the full diagnostic criteria for sarcopenia (Fig 1).
Fig 1.
Sarcopenia case-finding algorithm. Reproduced with permission from Cruz-Jentoft et al (2010).9
Sarcopenia is only one in a list of conditions associated with prominent muscle wasting, including malnutrition and cachexia. These conditions interact so significantly that defining them as different entities is a complex task, but careful differential diagnosis helps when choosing appropriate management.15 Starvation causes a loss of body fat and non-fat mass due to inadequate intake of protein and energy, while body fat in people with sarcopenia is preserved or increased (sarcopaenic obesity). Cachexia is severe muscle wasting (fat and non-fat mass) that accompanies disease states such as cancer. Definitions of cachexia have also been evolving in the past decade, but most definitions include the concept of inflammation as a leading mechanism underlying muscle wasting. Thus, most individuals with cachexia also have sarcopenia, but most sarcopenic individuals are not considered to be cachectic.
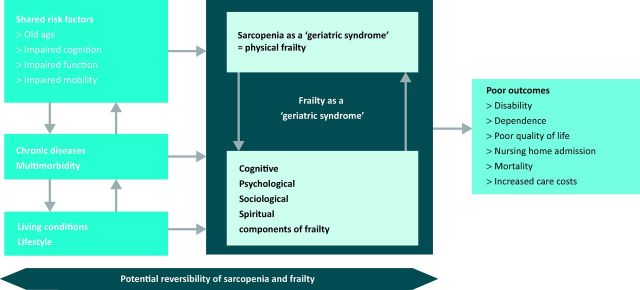
Physical frailty is another developing concept in geriatric practice and is strongly linked to sarcopenia. Frailty is a consequence of age-related cumulative declines across multiple physiological systems, with impaired homeostatic reserve and increased vulnerability to adverse health outcomes. The conditions of frailty and sarcopenia frequently overlap; most frail older people exhibit sarcopenia, and some older people with sarcopenia are also frail (Fig 2).16 Three of the five items proposed to identify the phenotype of frailty define sarcopenia by themselves. The relative benefits of using frailty as opposed to sarcopenia in clinical practice have yet to be determined.
Fig 2.
Sarcopenia and frailty. Reproduced with permission from Cruz-Jentoft (2013).16
Box 1. Criteria for the diagnosis of sarcopenia.
A syndromic approach
Sarcopenia should be considered as a geriatric syndrome rather than a classic medical syndrome.17 Geriatric syndromes have been defined as common, complex and costly states of impaired health in older individuals, which result from incompletely understood interactions of disease and age on multiple systems producing a constellation of signs and symptoms. A comprehensive multidimensional approach to investigate the aetiology of sarcopenia in affected individuals helps to disentangle the treatable and non-treatable risk factors and contributing conditions – much in the same way as delirium and falls are managed in geriatric practice.
Many of the most relevant causes of sarcopenia are listed in Table 1. However, special emphasis should be placed on lifestyle, especially nutrition and physical activity, as problems with each of these are usually prominent in most individuals with sarcopenia. Furthermore, specific nutritional approaches and physical exercise programmes represent the most efficient interventions to prevent and treat sarcopenia.
Interventions
Nutrition and physical exercise are the cornerstones of intervention in sarcopenia.18 Resistance exercise training increases muscle strength and mass and improves protein accretion in skeletal muscles. Aerobic exercise training may also benefit ageing skeletal muscle and improve insulin sensitivity. Exercise has to be prescribed and is most probably beneficial when properly supervised and sustained over time.
Correction of nutritional deficits is also needed. Caloric intake should be increased to cover increased demands posed by exercise. Protein requirements are also increased, with recommended intakes of proteins in sarcopenic patients of >1.2 g of protein per kilogram of body weight per day, except in patients with significant renal failure. Leucine, β-hydroxy β-methylbutyrate (HMB), creatine and some milk-based proteins may have beneficial effects on protein balance in skeletal muscle. Correction of vitamin D deficiencies is needed for proper muscle function, but the role of vitamin D in the presence of normal blood levels is yet to be determined.
No drug is currently approved for the treatment of sarcopenia.19 Studies with anabolic hormones have been disappointing. The next generation of drugs is directed at exploring inhibition of myostatin and manipulation of the neuromuscular junction.
References
- 1.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutrition 1997;127:990S–1S. [DOI] [PubMed] [Google Scholar]
- 2.Clark BC, Manini TM. Sarcopenia = / = dynapenia. J Gerontol A Biol Sci Med Sci 2008;63:829–34. 10.1093/gerona/63.8.829 [DOI] [PubMed] [Google Scholar]
- 3.Frontera WR, Zayas AR, Rodriguez N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Phys Med Rehabil Clin N Am 2012;23:201–7. 10.1016/j.pmr.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhanushaly M, Conwit R, Metter J, Ferrucci L. The role of the nervous system in muscle atrophy. In: Cruz-Jentoft AJ, Morley JE. (eds), Sarcopenia. Chichester: John Wiley & Sons, 2013:41–58. [Google Scholar]
- 5.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med 2008;24:455–69. 10.1016/j.cger.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 6.Mijnarends DM, Meijers JM, Halfens RJ, et al. Validity and reliability of tools to measure muscle mass, strength, and physical -performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc 2013;14:170–8. 10.1016/j.jamda.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Bijlsma AY, Meskers CG, Ling CH, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–81. 10.1007/s11357-012-9384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–9. 10.1016/j.jamda.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013;42:378–84. 10.1093/ageing/afs197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci 2012;67:48–55. 10.1093/gerona/glr035 [DOI] [PubMed] [Google Scholar]
- 12.Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–6. 10.1016/j.jamda.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012;31:652–8. 10.1016/j.clnu.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Morley JE, Cruz-Jentoft AJ. Definitions of sarcopenia. In: Cruz-Jentoft AJ, Morley JE. (eds), Sarcopenia. Chichester: John Wiley & Sons, 2013:8–19. [Google Scholar]
- 15.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr 2007;26:389–99. 10.1016/j.clnu.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 16.Michel JP, Cruz-Jentoft AJ. Sarcopenia: a useful paradigm for physical frailty. Eur Geriatr Med 2013;4:102–5. 10.1016/j.eurger.2013.02.009 [DOI] [Google Scholar]
- 17.Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care 2010;13:1–7. 10.1097/MCO.0b013e328333c1c1 [DOI] [PubMed] [Google Scholar]
- 18.Forbes SC, Little JP, Candow DG. Exercise and nutritional -interventions for improving aging muscle health. Endocrine 2012;42:29–38. 10.1007/s12020-012-9676-1 [DOI] [PubMed] [Google Scholar]
- 19.Landi F, Rolland Y, Onder G. The future of drug treatments. In: Cruz-Jentoft AJ, Morley JE. (eds), Sarcopenia. Chichester: John Wiley & Sons, 2013:296–323. [Google Scholar]