Key points
Obstructive sleep apnoea (OSA) is very common in patients with cardiovascular disease (CVD) and is associated with a worse prognosis
Patients with CVD should be screened for sleep apnoea
CVD risk should be measured and addressed in all patients with OSA
Compliance with continuous positive airway pressure treatment may reduce cardiovascular morbidity
Substantial weight loss can cure sleep apnoea and reduce cardiovascular risk
The sleep apnoea syndromes (SAS) comprise three main disorders:
obstructive sleep apnoea (OSA)
central sleep apnoea (CSA)
Cheyne-Stokes respiration (CSR).
CSA and CSR occur mainly in patients with established cardiovascular disease, while the main risk for OSA is obesity. OSA patients often have high cardiovascular risk – smoking, type 2 diabetes, hypertension and lipid abnormalities being common. Indeed, the obesity pattern associated with OSA (truncal obesity) is itself associated with higher cardiovascular risk.
As the prevalence of obesity increases so does OSA; thus, historical data for prevalence of OSA (2–4% for adult males)1 are likely to be an underestimate. For an average 0.5 million population, current estimates suggest at least 500 new referrals for OSA are likely per year with 200 new patients needing treatment with continuous positive airway pressure (CPAP).2
Early epidemiological studies were confounded by the strong associations between OSA and established cardiovascular risk markers, making it difficult to demonstrate an independent contribution from sleep apnoea. There is now a large volume of work linking sleep apnoea and cardiovascular disease. This link is important: since sleep apnoea is so prevalent, it may contribute to the enormous burden of cardiovascular morbidity and mortality.
OSA causes sleepiness, which reduces quality of life, and is also associated with a sixfold rise in risk of road traffic accident. CPAP is an established cost-effective treatment which improves sleepiness and quality of life in people with moderate to severe OSA.3 If CPAP treatment improves cardiovascular outcome in addition to current primary and secondary prevention measures, this should prompt a more active screening process for sleep apnoea in patients with cardiovascular disease.
Pathophysiology
It has been hypothesised that snoring vibrations might damage carotid vessel walls inducing plaque formation.4 Perhaps 30–40% of snorers have occasional OSA where the pharynx collapses during inspiration, obstructing airflow. Respiratory effort continues, generating large negative intrathoracic pressure swings. This raises left ventricular (LV) transmural pressure and afterload, increasing cardiac work at a time when the apnoea causes hypoxia, hypercapnia and sympathetic activation. After a variable time the patient arouses, reopens the airway and breathing resumes. At arousal, sympathetic activation raises heart rate and blood pressure (BP) sometimes by as much as 60 mmHg.5 The intrathoracic pressure swings are less in CSA because the apnoeas occur through altered respiratory drive. However, the same powerful physiological stressors are present which can adversely affect cardiac function.
It is easy to see how the hypoxia, hypercapnia and sympathetic activation occurring at a time of peak myocardial oxygen demand might adversely affect the heart, promoting regional ischaemia, arrhythmias or plaque destabilisation. There are wider consequences throughout the vascular tree, with OSA increasing systemic inflammation,6 triggering endothelial and circulating cells to release inflammatory cytokines,7 adhesion molecules and growth promoters. Sympathetic activation alters lipid and glucose metabolism, increasing free radical production and endothelial injury, vasoconstriction vessel remodelling, increasing vessel wall stiffness and platelet aggregation. These processes may promote a vicious circle of deteriorating endothelial, small and large vessel function including the heart and great vessels. Many of these inflammatory pathways have shown improvements with CPAP treatment.7
Hypertension
A number of epidemiological longitudinal studies have confirmed an independent relationship between OSA and both the prevalence and incidence of hypertension.8,9 Increasing severity of sleep apnoea is associated with increased likelihood of hypertension. OSA increases BP variability, raises BP during the night and daytime, is associated with a lack of nocturnal dip in BP and is often found in drug-resistant hypertension. CPAP treatment reduces BP by about 2–5 mmHg.10 CPAP, may be more effective in reducing BP in those with severe disease with sleepiness, and improved CPAP compliance potentially predicts a better response.11
Coronary arterial disease
OSA is linked to increased coronary artery calcification, prevalence of myocardial infarctions and incidence of coronary artery disease (CAD) and cardiovascular mortality.12,13 In CAD, OSA is associated with worse outcomes following primary and elective angioplasty:14
reduced recovery of myocardium and ejection fraction
increased risk of re-stenosis
the need for coronary bypass grafting
death.
Better outcomes are associated with CPAP treatment. If it is tolerated, CPAP is linked to reduced nocturnal angina, episodes of ST segment depression, future acute coronary syndromes, revascularisation procedures, admissions and cardiovascular deaths.15
Despite the strong circumstantial evidence, as yet there is no published, large, randomised controlled trial concerning reduced cardiovascular risk with the use of CPAP although several are underway.
Arrhythmias
A number of arrhythmias occur in SAS incidentally or in response to cardiac interventions. Heart rate varies through each individual cycle of apnoea. Heart rate variability is increased in SAS and can be used to screen for SAS. As arousal terminates an apnoea, resumption of ventilation leads to a tachycardia via sympathetic activation and changes in venous return. The attendant BP surge causes baroreflex-mediated bradycardia via the vagus nerve, with significant cardiac pauses reported frequently in patients with otherwise normal sino-atrial node activity.
Atrial fibrillation (AF) is very common in heart failure with Cheyne-Stokes breathing, and can also be triggered by OSA.16 Ventricular tachycardia and complex ventricular ectopy are described, mainly in OSA, with arrhythmia frequency increasing with worse hypoxaemia. CSR can trigger malignant arrhythmias, and is also associated with increased frequency of appropriate shock delivery in patients with severe cardiac failure and implanted defibrillators. Studies of patients with long-term ECG monitoring confirm that the arrhythmias are triggered by apnoeic events. CPAP treatment reduces both arrhythmia frequency overall and recurrence of AF following DC cardioversion.17
Heart failure
Sixty per cent of people with heart failure will have OSA, CSA or CSR. Conversely, LV impairment, particularly diastolic dysfunction, is common in people with OSA. Notably, even children naïve to standard cardiovascular risks demonstrate ventricular hypertrophy in association with OSA.18
In patients with confirmed heart failure, SAS is commoner in men. CSR is associated with increased age, AF, severe heart failure, raised pulmonary capillary wedge pressure and low daytime PaC02.19 OSA is associated with increased body mass index. Both OSA and CSA/CSR may occur in the same patient through a single night. On lying recumbent, peripheral oedema shifts centrally. In the lungs this alters capillary wedge pressure and respiratory drive, and in the upper airway, oedema may promote obstruction.
OSA is associated with incident heart failure in men.12 Prognosis is worse in heart failure patients with SAS, possibly through increased sympathetic activity. Control of LV function with cardiac resynchronisation therapy or heart transplantation can cure central sleep apnoea and is associated with improved prognosis. CPAP in patients with heart failure and OSA can improve quality of life, reduce arrhythmias and improve ejection fraction.20 A large randomised controlled trial of CPAP in patients with heart failure and CSA demonstrated no benefit in mortality overall, but there was some suggestion that mortality improved in patients in whom CSA was controlled.21 Trials of more complex ventilators are underway looking at mortality end-points in heart failure with sleep apnoea.
In the acute setting, patients may present with decompensated LV failure with pulmonary oedema. Alongside the standard cardiac treatments, CPAP and non-invasive ventilation (NIV/BILEVEL) can be used if initial treatment with high-flow oxygen via re-breathe mask fails to improve hypoxaemia.22 CPAP set at 10 cm water pressure should be delivered with supplemental oxygen to increase alveolar partial pressure of oxygen, improve recruitment of alveoli, offload respiratory muscles and help to clear lung water. NIV can be used for patients in type 2 respiratory failure. In this situation NIV is best delivered by oxygen-driven devices in a high dependency setting where blood gas monitoring, close supervision and ventilator adjustment are possible.
Fig 1.
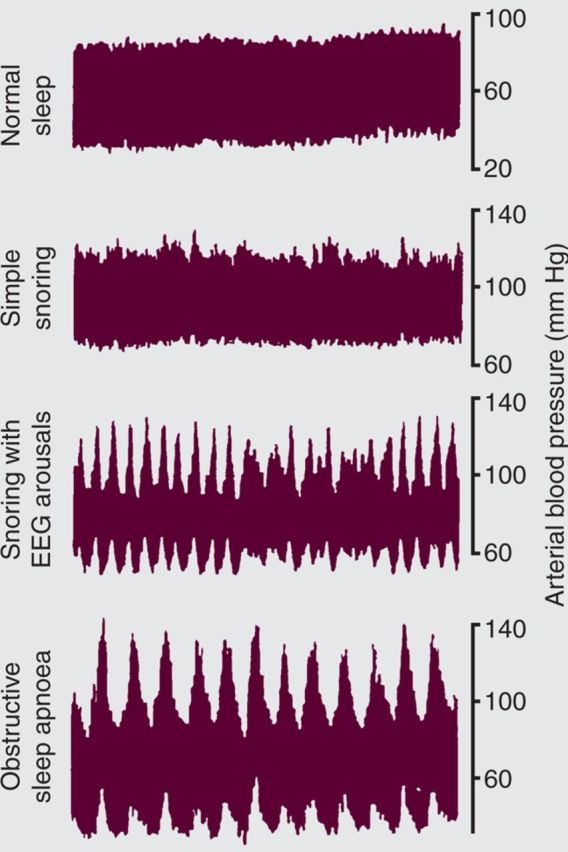
Blood pressure swings measured by Finapres non invasive finger plethysmography during sleep. Showing a progressive increase in blood pressure variability through snoring to obstructive sleep apnoea.
Alternatively, patients may be admitted in the acute setting with cor pulmonale and generalised oedema secondary to a combination of chronic hypoventilation, obesity and coexistent lung disease, cardiac and renal disease. Decompensation may be gradual or precipitated by sedative use or concurrent infection. In this setting, NIV can be used over several days, in combination with standard treatments, to improve blood gases and ventilatory drive and help offload the oedema.
Stroke
OSA is a risk factor for stroke.23 SAS are common after stroke, occurring in equal measure with haemorrhagic and thrombotic stroke and in any territory. They can be obstructive, central or mixed. Sleep apnoea conveys a poor prognosis after stroke; this can be improved by CPAP, although facial droop and poor compliance may limit its use. In the longer term, CPAP can reduce the incidence of further cardiovascular events in stroke patients.24
Death
Sleep apnoea increases mortality independent of the common confounders for risk of cardiovascular disease. In observational studies CPAP, if tolerated, improves overall mortality and cardiovascular disease-related mortality, returning risk to that of a normal population13 — although as yet no large prospective trials of CPAP have confirmed this. Since CPAP is so effective in controlling symptoms of daytime sleepiness, no such trial is likely to be performed in symptomatic patients. A number of studies are ongoing or due to report on whether CPAP might reduce risk in asymptomatic subjects.
Summary
Management of SAS and cardiovascular disease risk should be closely linked. It is important to screen for cardiovascular disease risk in patients with SAS and vice versa. CSA/CSR may be improved by ventilation strategies in heart failure, but benefit remains to be proven. For OSA, although CPAP may reduce cardiovascular disease risk, its main benefit is symptom control. In the longer term, CPAP should be used alongside standard cardiovascular risk reduction strategies including robust weight management programmes, with referral for bariatric surgery in appropriate cases. CPAP and NIV should be considered for acute admissions with decompensated cardiac failure.
Conflict of interest
JCP is involved in research in the field of sleep medicine, including a commercially funded study of adaptive servo-ventilation in central sleep apnoea with heart failure, sponsored by ResMed. He has received sponsorship to attend and lecture at scientific meetings from Astra Zeneca, Glaxo Smith Kline and Resmed.
References
- 1.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax 1991;46:85–90 10.1136/thx.46.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.British Thoracic Society Service specification for investigation and treatment of obstructive sleep apnoea syndrome. London: BTS, 2010. [Google Scholar]
- 3.National Institute for Health and Clinical Excellence Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea. London: NICE, 2008. [Google Scholar]
- 4.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep 2008;31:1207–13 [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley TD, Phillipson EA. Pathogenesis and pathophysiology of the obstructive sleep apnea syndrome. Med Clin North Am 1985;69:1169–85 [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep 2007;30:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002;165:934–9 [DOI] [PubMed] [Google Scholar]
- 8.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829–36 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–84 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 10.Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;3:CD001106. [DOI] [PubMed] [Google Scholar]
- 11.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006;27:1229–35 10.1183/09031936.06.00062805 [DOI] [PubMed] [Google Scholar]
- 12.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19–25 [DOI] [PubMed] [Google Scholar]
- 13.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–53 [DOI] [PubMed] [Google Scholar]
- 14.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol 2007;99:26–30 10.1016/j.amjcard.2006.07.055 [DOI] [PubMed] [Google Scholar]
- 15.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol 2007;50:1310–4 10.1016/j.jacc.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364–7 10.1161/01.CIR.0000136587.68725.8E [DOI] [PubMed] [Google Scholar]
- 17.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–94 [DOI] [PubMed] [Google Scholar]
- 18.Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med 2002;165:1395–9 10.1164/rccm.2105118 [DOI] [PubMed] [Google Scholar]
- 19.Solin P, Bergin P, Richardson M, et al. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation 1999;99:1574–9 10.1161/01.CIR.99.12.1574 [DOI] [PubMed] [Google Scholar]
- 20.Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004;169:361–6 10.1164/rccm.200306-752OC [DOI] [PubMed] [Google Scholar]
- 21.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007;115:3173–80 10.1161/CIRCULATIONAHA.106.683482 [DOI] [PubMed] [Google Scholar]
- 22.Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med 2010;152:590–600 [DOI] [PubMed] [Google Scholar]
- 23.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005;172:1447–51 10.1164/rccm.200505-702OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Garcia MA, Soler-Catalu a JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischaemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009;180:36–41 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]


