Key points
Narcolepsy is under-recognised and should be considered in cases of unexplained daytime sleepiness or collapse
Narcolepsy has significant adverse effects on quality of life and socioeconomic impacts
Accurate diagnosis requires careful consideration of clinical and electrophysiological features
Effective drug treatments are available
Exclusion of classical narcolepsy should prompt the consideration of monosymptomatic or secondary narcolepsy, and idiopathic hypersomnolence because treatment can still be effective
Narcolepsy with cataplexy is primarily a disorder of excessive daytime sleepiness (EDS) and episodic collapse. Despite a quality of life impact similar to Parkinson's disease (PD)1 and significant individual and societal costs2 it is often overlooked. A UK survey found median time from first symptom to diagnosis to be 10.5 years.3 The delay in diagnosis may be because it is uncommon (European prevalence 0.047%4), but is no doubt exacerbated by a paucity of sleep medicine training (median 5 min in UK medical undergraduate curricula5). Narcolepsy has a variety of presentations which may be wrongly attributed to more familiar disorders or lifestyle and there is an overlap with normality. EDS is reported by up to 10% of the general population6 and objective measures of sleepiness do not reliably differentiate narcoleptics and normal subjects.7 It is important for the acute care physician to be aware of the features of, and means for, diagnosing narcolepsy so that opportunities to treat this disabling condition are not missed.
Diagnosis
Anyone reporting EDS warrants a detailed sleep history. It is important to tease out an increased propensity to fall asleep from other symptoms reported as tiredness, particularly physical and emotional fatigue. The Epworth Sleepiness Scale can be a useful measure.8 Explanations for perceived sleepiness, including lifestyle, mood disorders, other medical conditions and medication, should be explored (Table 1). People with narcolepsy typically need brief, refreshing naps; if these are not possible, they can have irresistible sleep episodes (‘sleep attacks’). The identification of other factors such as sleep disordered breathing (SDB) contributing to sleepiness should not discourage the pursuit of a diagnosis of narcolepsy if features are compelling because sleep disorders can coexist.9
Table 1.
Causes of excessive daytime sleepiness.

Cataplexy
Cataplexy is emotionally triggered muscle weakness and, with EDS, is essential to the diagnosis of classical narcolepsy.9–11 Symptoms usually develop months or years after EDS but, because sleepiness can be overlooked, cataplexy is sometimes the presenting complaint. It should be considered when patients present with apparent absence episodes, unexplained collapse or episodic clumsiness. Prolonged or intractable episodes (status cataplecticus) may be mistaken for seizures, coma or malingering. The weakness of cataplexy is typically bilateral and can involve the legs (‘weak at the knees’), hands, face, neck or shoulder girdle. Frequency can range from rarely to several times a day. Ptosis may give the impression of unconsciousness and distract from the correct diagnosis but, apart from occasional evolution into sleep, awareness is preserved.
The differential diagnosis of cataplexy includes periodic paralysis, myasthenia gravis, atonic seizures and vertebrobasilar insufficiency.10 The absence of cataplexy can represent disease-in-evolution or so-called ‘monosymptomatic narcolepsy’9 but other diagnoses should be considered.
Other narcoleptic symptoms
The other symptoms of the ‘narcoleptic tetrad’ involve different facets of rapid eye movement (REM) sleep invading wakefulness:
sleep paralysis (persistent motor atonia)
hypnogogic/hypnopompic hallucinations (dream-like experiences during sleep-wake transitions).
Automatism, poor nocturnal sleep and dream enactment (REM sleep behaviour disorder) can also occur.9–11
Although not inevitable in, or exclusive to, narcolepsy, when accompanying cataplexy and sleep attacks, it is understandable how lack of familiarity with these features can result in narcolepsy being mistaken for seizures, psychiatric disorders or malingering.
Physical examination
The physical examination is usually normal. Cataplexy has characteristic features, including areflexia and facial twitching, but episodes usually occur only in relaxed, intimate settings and so are rarely seen in clinic.11 Unless the diagnosis can be confidently made on clinical features alone, suspected narcolepsy and other unexplained cases of EDS should undergo specific investigation.9
Pathophysiology
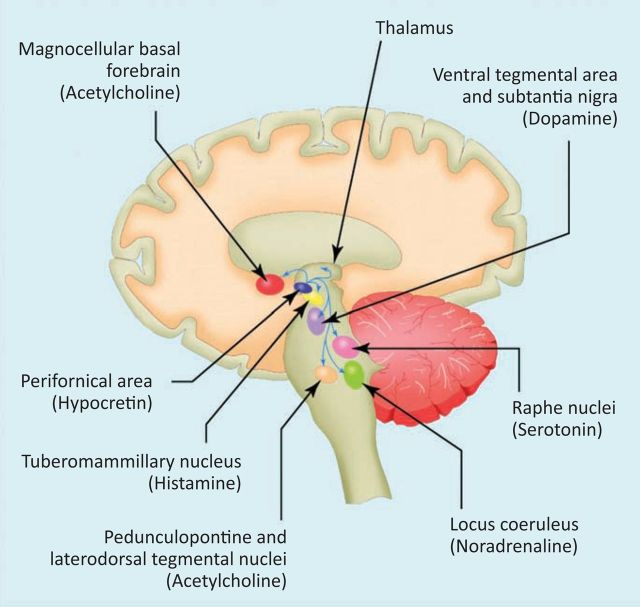
The discovery of the neurotransmitter orexin (hypocretin) has refined the pathophysiological description of narcolepsy as a condition of sleep-wake instability.12 Orexin stabilises wake and sleep states through widespread subcortical neuronal projections from the lateral hypothalamus (Fig 1). Cerebrospinal fluid (CSF) and brain studies have established that most people with narcolepsy and cataplexy have orexin deficiency.14–16
Fig 1.
Hypocretin (orexin) system. The sleep-wake cycle is governed by a complex, multilevel neuronal system in the brainstem, thalamus, hypothalamus and basal forebrain. Hypocretin-producing neurons in the hypothalamus stabilise the activity of other key neuronal groups involved in the control of sleep and waking. These nuclei and their principal neurotransmitters are shown here in highly schematic fashion. Reproduced with permission from BMJ Publishing Group Ltd.13
Up to 2% of narcoleptics have symptomatic first-degree relatives but most cases are sporadic.17 Global research has yielded only one case of human narcolepsy caused by a prepro-orexin gene mutation15 but wider genetic studies continue. There is a strong human leukocyte antigen (HLA) association. The DQB1∗0602 haplotype is found in 88–98% of patients with non-familial cataplexy.18 The newly discovered association of narcolepsy with a T cell receptor polymorphism and demonstration of elevated antistreptococcal antibodies in recent-onset cases support the hypothesis of a genetically determined autoimmune condition triggered by infection.19,20
Investigation
Nocturnal polysomnography
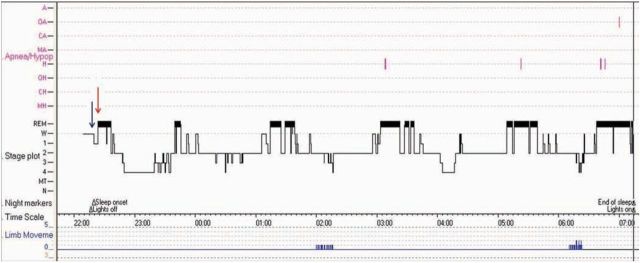
The investigation of choice for unexplained EDS is nocturnal polysomnography (PSG). Electroencephalogram, chin electromyogram and electro-oculogram enable the staging of wakefulness, REM and non-REM sleep. Respiratory and leg monitoring allow screening for SDB and periodic limb movement. Features suggestive of narcolepsy include rapid sleep onset and premature entry into REM sleep (Fig 2).
Fig 2.
Polysomnogram summary (hypnogram) from patient with narcolepsy. There is rapid sleep onset (blue arrow) and early rapid eye movement (REM) sleep (red arrow). There are clinically insignificant numbers of apnoeas (purple marks) and periodic limb movements (bottom trace in blue) which do not explain sleepiness in this case.
Multiple sleep latency test
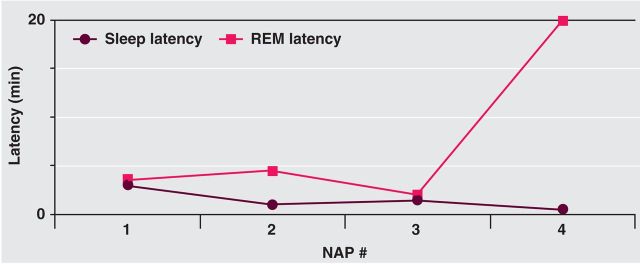
Diurnal sleep drive is measured by the multiple sleep latency test (MSLT). Subjects undergo PSG-monitored nap opportunities at two-hourly intervals.7 Mean sleep latency is usually less than eight minutes in narcolepsy (most narcoleptics fall asleep within 5 min)7 and multiple REM sleep episodes occur (Fig 3). International guidelines mandate a combination of these two criteria but sensitivity is only around 80% in classical cases and they are not entirely specific.9,21,22
Fig 3.
Characteristic narcoleptic multiple sleep latency test result showing evolution of latencies. The subject slept on each of four opportunities offered through the day. The mean sleep latency is only 1.5 min and rapid eye movement (REM) sleep is seen in three of the naps.
Other investigations
HLA testing has only limited utility as it is positive in up to 38% of non-narcoleptics.18 A negative test with a classical history may cause pause for thought. CSF orexin assay may be useful when PSG and MSLT are unavailable, but the test is invasive and less discriminatory in cases without cataplexy.9,14
Secondary narcolepsy and other hypersomnias
Rarely, other medical disorders present with narcoleptic features. Those featuring cataplexy include:
hypothalamic lesions
paraneoplastic encephalitis
Niemann-Pick type C disease
Coffin-Lowry syndrome.
In the absence of abnormalities on neurological examination there is no justification for neurological imaging. Narcolepsy-like sleepiness has been reported in:
head trauma
myotonic dystrophy
Prader-Willi syndrome
PD
multisystem atrophy.
To fulfil diagnostic criteria, patients should have the characteristic MSLT features with SDB excluded or controlled.9
Recurrent hypersomnias are rare and little understood. Kleine-Levin syndrome is the best characterised, with recurrent episodes of hypersomnolence, often first in adolescence, which may last several days and be interspersed with asymptomatic intervals of weeks to years. Cognitive and behavioural abnormalities, classically binge-eating and hypersexuality, are variably associated. A prolonged 24-hour sleep time may be demonstrated if PSG is achieved during an episode.9 Lithium may be useful, but evidence for its effectiveness is limited.23
In patients with unexplained EDS, idiopathic central nervous system hypersomnolence (ICNSH) should be considered. This diagnosis has been inconsistently described and ascribed.24 The new International Classification of Sleep Disorders has attempted to address this inconsistency with diagnostic criteria9 but, in the absence of any known aetiology or diagnostic test, these are arbitrary. There is typically difficulty waking up in the morning (sleep inertia) and EDS. Naps are more prolonged than in narcolepsy and usually unrefreshing. If nocturnal sleep duration allows time for the performance of MSLT after PSG, the mean sleep latency should be under eight minutes without associated multiple REM sleep episodes.9
Management
Lifestyle
Optimal sleep hygiene is important for any disorder with EDS. Nocturnal sleep should be sufficient and regular and the environment conducive to sleep. Alcohol and caffeine intake should be moderated. Brief naps are often useful in narcolepsy but rarely in ICNSH. Patients should be advised of the need to report their diagnosis to the driving authorities and not to drive until EDS is controlled.
Wakefulness-promoting therapy
Drug treatment for hypersomnolence is similar for narcolepsy and ICNSH, although the evidence base is stronger for narcolepsy.
Modafinil is recommended as first-line treatment for narcolepsy.23 Tolerance and dependency are not reported. Side effects are usually mild and rarely lead to discontinuation. Modafinil is used in ICNSH, although it may not be as effective as in narcolepsy; this use is not licensed in the UK.23,25 It may be an option in hypersomnia associated with PD and myotonic dystrophy.23
Dexamfetamine. Dexamfetamine (5–60 mg/day), methylphenidate (5–100 mg/day) and other stimulants have long been used to treat narcolepsy and ICNSH. They can be very effective, although there is potential for toler ance and dependency. Sympathomimetic side effects are recognised and higher doses have been associated with psychosis.24 Risk-benefit data are scanty and these drugs are usually second-line therapy.23
Anticataplectics
If cataplexy requires treatment, the REM sleep-suppressing tricylic antidepressants and selective serotonin reuptake inhibitors have an established but poorly evidenced role. They can also be useful in sleep paralysis and hallucinations. Alternatives include venlafaxine and reboxetine.23 Rebound cataplexy can occur with drug withdrawal.
Sodium oxybate
Sodium oxybate is effective in narcolepsy and cataplexy23 although its mode of action is not clear. It is a profound sedative, promoting deep non-REM (slow wave) sleep. Its rapid onset of action and short half-life require it to be taken at night in divided doses. These are titrated up over several weeks until symptoms are controlled, maximum dose reached (9 g) or side effects preclude further increase. Full benefit takes longer to manifest. There is no tolerance, dependency or withdrawal syndrome. Often used first-line in the USA, its expense means it is reserved for refractory cases in the UK where funding requires individual case approval.
Conclusions
It is important to consider the possibility of narcolepsy and other non-respiratory disorders in patients with EDS. Accurate and timely diagnosis should be the norm. Symptoms can be disabling and costly but effective treatment is available.
Conflict of interests
TQ has received sponsorship from UCB Pharma Ltd and a travel bursary from Cephalon UK to attend sleep medical conferences. IES has received a speaker's fee from Cephalon UK and sponsorship from UCB Pharma Ltd to attend a sleep medical conference.
References
- 1.Beusterien KM, Rogers AE, Walsleben JA, et al. Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep 1999;22:757–65 [DOI] [PubMed] [Google Scholar]
- 2.Dodel R, Peter H, Walbert T, et al. The socioeconomic impact of narcolepsy. Sleep 2004;27:1123–8 [DOI] [PubMed] [Google Scholar]
- 3.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004;5:37–41 10.1016/j.sleep.2003.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 2002;58:1826–33 10.1212/WNL.58.12.1826 [DOI] [PubMed] [Google Scholar]
- 5.Stores G, Crawford C. Medical student education in sleep and its disorders. J R Coll Physicians Lond 1998;32:149–53 [PMC free article] [PubMed] [Google Scholar]
- 6.Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. Am J Pub Health 1997;87:1649–53 10.2105/AJPH.87.10.1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005;28:113–21 [DOI] [PubMed] [Google Scholar]
- 8.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5 [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine Diagnostic and coding manual. International classification of sleep disorders, 2nd edn. Westchester, IL: AASM, 2005. [Google Scholar]
- 10.Aldrich M. Narcolepsy and related disorders. In: Aldrich M Sleep medicine. New York: Oxford University Press, 1999:152–74 [Google Scholar]
- 11.Parkes JD, Chen SY, Clift SJ, Dahlitz MJ, Dunn G. The clinical diagnosis of the narcoleptic syndrome. J Sleep Res 1998;7:41–52 10.1046/j.1365-2869.1998.00093.x [DOI] [PubMed] [Google Scholar]
- 12.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003;53:154–66 10.1002/ana.10444 [DOI] [PubMed] [Google Scholar]
- 13.Zeman A, Britton T, Douglas N, et al. Narcolepsy and excessive daytime sleepiness. BMJ 2004;329:724–8 10.1136/bmj.329.7468.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 2002;59:1553–62 10.1001/archneur.59.10.1553 [DOI] [PubMed] [Google Scholar]
- 15.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000;6:991–7 10.1038/79690 [DOI] [PubMed] [Google Scholar]
- 16.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27:469–74 10.1016/S0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mignot E. Genetic and familial aspects of narcolepsy. Neurology 1998;50:S16–22 10.1212/WNL.50.2_Suppl_1.S16 2 Suppl 1 [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Hungs M, Mignot E. Narcolepsy and the HLA region. J Neuroimmunol 2001;117:9–20 10.1016/S0165-5728(01)00333-2 [DOI] [PubMed] [Google Scholar]
- 19.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet 2009;41:708–11 10.1038/ng.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aran A, Lin L, Nevsimalova S, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep 2009;32:979–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep 1997;20:620–9 [PubMed] [Google Scholar]
- 22.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain 2006;129:1609–23 10.1093/brain/awl079 [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007;30:1705–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali M, Auger RR, Slocumb NL, Morgenthaler TI. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med 2009;5:562–8 [PMC free article] [PubMed] [Google Scholar]
- 25.Mitler M, Aldrich MS, Koob GF, Zarcone VP. Narcolepsy and its treatment with stimulants. ASDA standards of practice. Sleep 1994;17:352–71. [PubMed] [Google Scholar]