Abstract
Background:
Depression has been reported in patients with chronic rhinosinusitis (CRS), but its prevalence varies across studies, and uncertainty remains regarding the association with baseline disease severity and treatment outcomes.
Objective:
To systematically assess the prevalence of depression in CRS and to review its relationship to baseline disease severity and outcomes after treatment.
Methods:
A systematic review of the prevalence of possible depression was performed by using the available methods to diagnose depression, and the results were pooled. Studies that examined the relationship of depression on baseline disease severity and treatment outcomes were organized and reported individually.
Results:
Thirteen studies met inclusion criteria for prevalence analysis. The prevalence of possible or likely depression in patients with CRS ranged from 11.0 to 40.0%, depending on the method of diagnosis and sensitivity of various depression instruments. Positive depression screening was consistently associated with worse CRS-specific quality of life (QOL), medication usage, and health care utilization, but there were no reliable CRS-specific factors to predict the presence of depression. Patients with possible depression who underwent medical or surgical treatment for CRS tended to have improvements in CRS-specific QOL but did not achieve the same degree of QOL as patients who were not depressed. Depression-specific QOL seemed to improve after treatment for CRS.
Conclusion:
Positive depression screening was common in patients with CRS and had a negative association on the entire spectrum of QOL, health care utilization, and productivity. CRS-specific treatments were still beneficial in patients who seemed to be depressed and improved both depression-specific and CRS-specific QOL.
Keywords: Chronic rhinosinusitis, depression, productivity, quality of life, polyp
The prevalence of physician-diagnosed depression in the United States is nearly 9%1 and is the leading cause of disability among adults in high income countries.2 Depression has a major effect on quality of life (QOL) and economic burden, with an estimated lost productivity cost of $23 billion in 2011.3 In addition to major depression as a primary disorder, comorbid depression has been found in a variety of other chronic medical illnesses4 and often remains underdiagnosed because physicians and patients focus on the primary disease.
The presence of comorbid depression in chronic medical illness is often associated with negative health outcomes. For example, when comorbid depression is present in chronic obstructive pulmonary disease, it is associated with worse QOL, increased health care utilization, and even increased mortality.5 After coronary artery bypass, patients with depression have increased mortality and rehospitalization.4 Although the potential bidirectional nature of comorbid depression and poor outcomes makes causality difficult to determine, its strong association with negative outcomes is clinically relevant, both from a prognostic and treatment standpoint.
Similar to other chronic illnesses, there is a reason to suspect that patients with chronic rhinosinusitis (CRS) have comorbid depression at a rate higher than population norms. However, estimating the true prevalence of comorbid depression and its potential impact in CRS is difficult for several reasons. First, patients with CRS are primarily treated by otorhinolaryngologists, who are experts in sinusitis management but who may not identify or address depressive symptoms. This reality is reflected in many CRS outcomes studies that fail to quantify depression. In addition, studies that depend only on a previous physician-given diagnosis of comorbid depression might underestimate the true prevalence of depression. Given preliminary reports of increased depression associated with CRS, it is critical for those who treat CRS to understand its true prevalence, as well as its impact on clinical presentation and treatment outcomes, to optimize care of our patients.
The goal of this review was to systemically evaluate the overall prevalence of depression in patients with CRS diagnosed both by physicians and through a variety of screening instruments. Secondary goals were to determine the association of comorbid depression on patients with CRS, potential factors that could alert clinicians to the presence of undiagnosed depression, and the relationship of comorbid depression on outcomes after treatment for CRS.
METHODS
Systematic Review of the Prevalence of Depression in CRS
Two reviewers (S.G., P.K.) independently performed a literature search by using PubMed (1947 to December 2015) and Scopus (1973 to November 2015) for studies that evaluated the prevalence of depression in the setting of CRS. The PubMed keywords and Medical Subject Heading terms used were “depression” or “depressive” and “rhinosinusitis” or “sinusitis” or “nasal” or “polyp.” Scopus (1999 to November 2015) was searched by using the terms “depression” or “depressive” and “rhinosinusitis” or “sinusitis” in the abstract and in the article title. Peer-reviewed articles in press were also included. Inclusion criteria required that all patients must have an explicit diagnosis of CRS by a physician. Self-reported CRS was excluded.
Depression assessments included a depression diagnosis by a previous physician or screening via validated questionnaires. Self-reported depression was excluded. Studies with mixed populations of patients with various sinonasal disorders were excluded unless data from patients with CRS could be isolated. Studies in which the frequency of depression was not provided or could not be deduced were also excluded. References from all identified studies were reviewed to determine if any additional articles were appropriate for inclusion. All articles were considered regardless of language. This study was considered exempt by the Medical University of South Carolina's institutional review board. The data from included studies were extracted and analyzed independently by two of us (S.G., Z.M.S.). The selected studies were categorized based on diagnostic method for depression. Categories included validated questionnaire and previous physician diagnosis. The combined prevalence of depression was calculated for each diagnostic method and reported as a summary frequency weighted by sample size.
Secondary Review of Depression Impacts
Data related to factors associated with depression in patients with CRS or the impact of comorbid depression on patient outcomes did not lend themselves to meta-analysis, given relative sparsity and heterogeneity. As such, these data were extracted from all the studies found during a previous literature search of depression in CRS, irrespective of whether they were included in prevalence estimates. Findings from these studies were tabulated and presented in an organized and descriptive fashion.
RESULTS
Systematic Review of a Positive Depression Screen Results in CRS
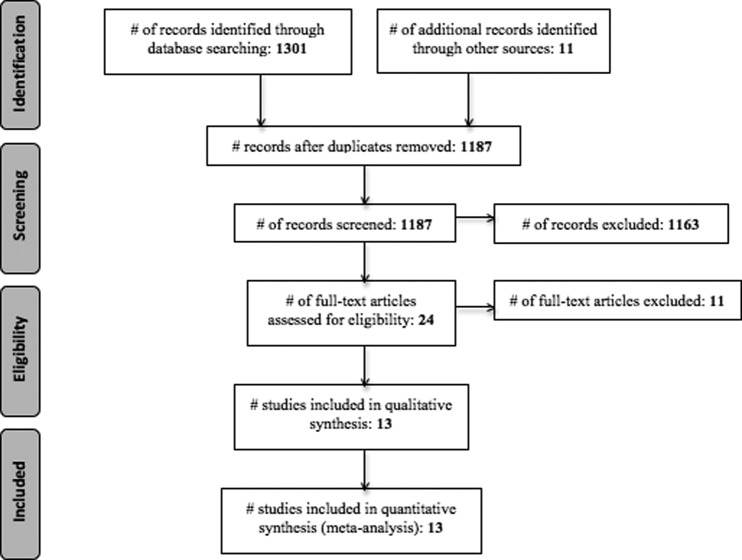
A total of 1187 articles were screened for eligibility, and 13 studies were selected based on inclusion-exclusion criteria. The search strategy with flow diagram is presented per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines6 (Fig. 1). These studies include a total of 2774 adult patients with CRS, with six studies that used the American Academy of Otolaryngology—Head and Neck Surgery criteria,7–12 four studies that used European Position Paper on Rhinosinusitis and Nasal Polyps criteria,13–16 two studies that used both the American Academy of Otolaryngology—Head and Neck Surgery and the European Position Paper on Rhinosinusitis and Nasal Polyps criteria,17,18 and one study that used physician diagnosis to determine CRS.19 With regard to depression, there were no studies that used strict Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria.20 Nine studies used a validated questionnaire to screen for depression,9,11,13–19 and four studies relied on a previous physician diagnosis.7,8,10,12
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
There were four studies that used physician-diagnosed depression (n = 631 patients).7,8,10,12 All of these studies relied on patient report or medical record confirmation of other non-otolaryngologist physicians to make the diagnosis of depression. Two studies also required the use of antidepressants or psychologic counseling.7,8 The combined prevalence of depression for studies that used physician-diagnosed depression was 18.1% (n = 114/631), with a range from 13.4 to 25% (Table 1).7,8,10,12 Validated patient-reported questionnaires used to diagnose depression in the selected studies included the Hospital Anxiety and Depression Score (HADS), the 2-item Patient Health Questionnaire (PHQ2), the 9-item Patient Health Questionnaire (PHQ9), (and the Beck Depression Inventory (BDI). All three questionnaires are self-reported scales used to determine the severity of depression by evaluating the frequency of depressive symptoms. Scoring can be variable, but, generally, the higher the score, the more severe the depression.
Table 1.
Prevalence of comorbid depression in patients with CRS
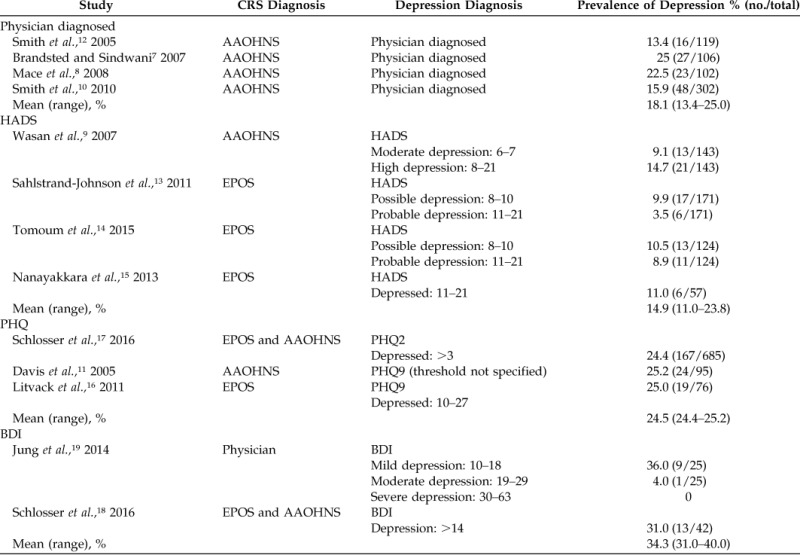
CRS = Chronic rhinosinusitis; AAOHNS = American Academy of Otolaryngology—Head and Neck Surgery; HADS = Hospital Anxiety and Depression Score; EPOS = European Position Paper on Rhinosinusitis and Nasal Polyps; PHQ = Patient Health Questionnaire; BDI = Beck Depression Inventory.
The HADS is a 14-item questionnaire used to determine severity of depression and anxiety by evaluating the frequency of depression and anxiety symptoms over a 1-week period. The seven questions related to depression are scored separately from the seven questions related to anxiety to give two individual scores. Four studies used the HADS questionnaire (n = 495 patients). The prevalence of depression varied, depending on the threshold used to define depression. The study that used the lowest cutoff, of ≥6, reported the highest prevalence, of 23.8%.9 Two studies used a cutoff of 8 points, which resulted in prevalences that ranged from 15.4 to 19.4%.13,14 The strictest study used ≥11 points as a cutoff and, not surprisingly, resulted in the lowest prevalence, of only 11%15 (Table 1). The overall prevalence of depression in CRS that used HADS was 14.9%, with a range from 11.0 to 23.8%, depending on the threshold used to define depression (Table 2).
Table 2.
Impact of comorbid depression on CRS presentation
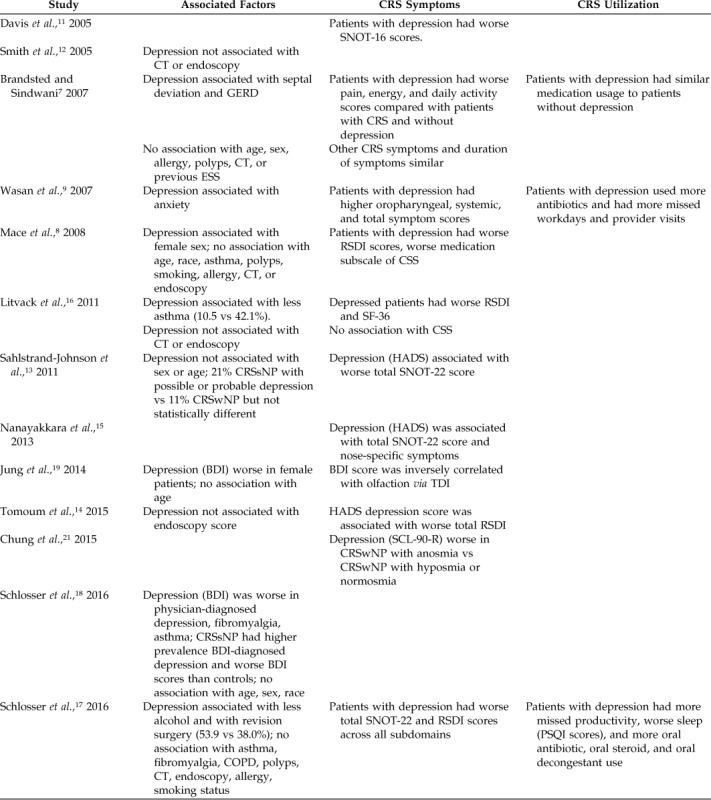
CRS = Chronic rhinosinusitis; SNOT-22 = 22-item Sino-Nasal Outcome Test; SNOT-16 = 16-item Sino-Nasal Outcome Test; CT = computed tomography; GERD = gastroesophageal reflux disease; ESS = endoscopic sinus surgery; RSDI = Rhinosinusitis Disability Index; CSS = chronic sinusitis survey; CRSsNP = chronic rhinosinusitis without nasal polyposis; HADS = Hospital Anxiety and Depression Score; BDI = Beck Depression Inventory; TDI = threshold, discrimination, identification (range 0–48); SCL-90-R =Symptom Checklist-90-Revised; CRSwNP = chronic rhinosinusitis with nasal polyposis; COPD = chronic obstructive pulmonary disease; PSQI = Pittsburgh Sleep Quality Index; SF-36 = short-form health survey.
The PHQ consists of two (PHQ2) or nine (PHQ9) questions by using a Likert scale, and patients are asked to consider the frequency of symptoms over a 2-week period. The only study to use PHQ2 was also the largest (n = 685 patients) and reported a prevalence of 24.4%17 (Table 1). Two studies used PHQ9, and both reported a prevalence of 25% (Table 1). Overall, 856 patients were studied by using the PHQ questionnaires with a prevalence of ∼25%, regardless of whether the two-item or nine-item version was used. The BDI consists of 21 multiple-choice questions, each scored from 0 to 3 for a total of 63 possible points. Two studies that fit the criteria for this systematic review used the BDI. The first study used a validated Japanese version of the BDI-II, which was revised from the original BDI in 1996 to correspond with the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria.19,20 A score of ≥10 was used to define depression, which resulted in a prevalence of mild depression to be 36%, moderate depression to be 4%, and severe depression to be 0%. The combined prevalence, therefore, was 40% (Table 1). The other BDI study used a cutoff of ≥14 to diagnose depression and found a prevalence of 31.0%.18 Among the nine studies that used validated questionnaires,9,11,13–19 the prevalence of depression ranged from 11.0 to 40.0%. As seen from both the HADS and BDI data, the score used to define depression varied among the studies, and the lower thresholds for defining depression resulted in higher prevalences (Table 1).
Factors Associated with a Positive Depression Screen Result in Patients with CRS
Given the high prevalence of depression in CRS, we then examined factors that may aid clinicians in detecting depression in their patients (Table 2). With regard to demographics, depression was associated with female sex in two studies,8,19 whereas three studies failed to detect an association.7,13,18 In general, other demographic factors, such as age and race, were uniformly not associated with depression. In addition, there were no comorbidities consistently associated with depression. Asthma and fibromyalgia results were mixed, whereas allergic rhinitis, chronic obstructive pulmonary disease, and smoking were not associated with depression in any study. Six studies7,8,12,14,16,17 examined CRS-severity factors by using endoscopy and/or computed tomography, and none found an association with depression. Reports on the impact of polyp status and revision surgery were mixed.
When depression is present in CRS, it is consistently associated with worse CRS-specific QOL as measured by a variety of instruments, including the Rhinosinusitis Disability Index, the 22-item Sino-Nasal Outcome Test, and the Chronic Sinusitis Survey. Three studies examined baseline medication usage, productivity, or physician visits, and found that comorbid depression was uniformly associated with worse performance across each of these metrics (Table 2).7,9,17 Two studies19,21 reported worse baseline olfaction in patients with CRS and depression, although one of these studies was limited to patients with CRS with nasal polyposis.21
Impact of Depression on Outcomes
Six studies report that CRS-specific metrics improved after endoscopic sinus surgery in patients with comorbid depression,8,10–12,16,17 whereas one study failed to find improvement7 in this population (Table 3). Whether patients with depression have the same degree of improvement after endoscopic sinus surgery as do patients without depression is unclear. The majority of studies indicated that patients with depression start and end with worse CRS-specific QOL than patients without depression but that their absolute level of improvement (i.e., change from baseline) is similar. One study examined medical therapy for CRS and found that it also improves 22-item Sino-Nasal Outcome Test in patients with depression, similar to endoscopic sinus surgery.17
Table 3.
Impact of depression on outcomes
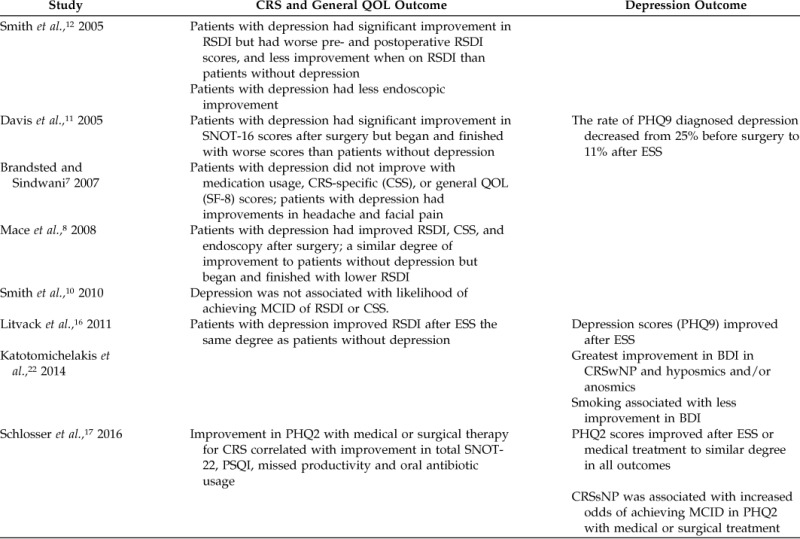
CRS = Chronic rhinosinusitis; QOL = quality of life; RSDI = Rhinosinusitis Disability Index; SNOT-22 = 22-item Sino-Nasal Outcome Test; SNOT-16 = 16-item Sino-Nasal Outcome Test; PHQ = Patient Health Questionnaire; ESS = endoscopic sinus surgery; CSS = chronic sinusitis survey; SF = short form; MCID = minimal clinically important difference; BDI = Beck Depression Inventory; CRSwNP = chronic rhinosinusitis with nasal polyposis; PSQI = Pittsburgh Sleep Quality Index; CRSsNP = chronic rhinosinusitis without nasal polyposis; SF-8 = 8-item short-form health survey.
Four studies examined depression-specific outcomes after CRS-specific medical or surgical therapy, and all found improvement in depression.11,16,17,22 These studies aimed to determine whether treatment of CRS specifically would impact comorbid depression secondarily. Overall, there are limited data, but it seems that treatment of CRS results in a mean improvement in depression scores. The greatest improvement in depression-specific outcomes seems to be in those with olfactory dysfunction and nonsmokers. The impact of nasal polyps was beneficial to depression outcomes in one study of patients with CRS with nasal polyposis,22 whereas another study found increased odds of achieving a minimal clinically important difference in patients with CRS without nasal polyposis.17 The data that support depression-specific improvement are limited, and most studies were not designed to investigate this outcome in a comprehensive fashion.
DISCUSSION
Depression seems to be a comorbid condition in CRS, with a prevalence of up to 40%. The criterion standard for diagnosing depression involves detailed evaluation by a mental health expert by using Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria.20 Unfortunately, we were unable to find any studies that used such strict diagnostic criteria but had to rely on screening instruments and previous physician diagnosis of depression that may aid otolaryngologists in identifying this important comorbidity. We found that, as screening instruments become more detailed and as thresholds for defining depression are lowered, they detect an increasing prevalence of possible depression, above and beyond that seen based solely on existing physician diagnosis. This finding indicated that comorbid depression was likely undiagnosed and underappreciated in this patient population.
The U.S. Preventive Services Task Force recommends screening and treatment of depression in primary care settings with PHQ or HADS instruments.2 O'Connor et al.23 report that screening instruments demonstrate sensitivity of 80–90% and specificity of 70–85%. This obviously varies widely, depending on the demographics of the population being studied, the screening instrument used, and the thresholds used for each of the various instruments. The expected rate of depression in a general primary care setting is much lower than what we found across CRS populations, which indicated that routine screening of the CRS population would have even greater utility. Although U.S. Preventive Services Task Force recommendations were not specific to CRS populations, it was apparent that some sort of screening would be worthwhile to help identify undiagnosed depression.2 Further work is clearly needed to determine the optimal screening tool and ideal thresholds that balance sensitivity and specificity in a CRS population, confirmed by psychiatric evaluation. However, simple two-item questionnaires, e.g., the PHQ2, seem to be a reasonable start for screening. Use of this instrument can be efficiently integrated into intake questionnaires both at baseline and follow-up time points. Although screening instruments are not sufficient for a definitive diagnosis of depression, those with positive screening results can be referred to a mental health expert for a more detailed assessment and determination of depression treatment.23
When positive depression screening results occur in patients with CRS, all the studies demonstrated a negative association with CRS-specific QOL. Impaired global health outcomes even extended to medication usage, productivity, and physician visits. The true economic impact of comorbid depression in CRS is unknown but is likely to be millions of dollars each year. Unfortunately, there were no demographic, comorbid, or CRS-specific factors that reliably predicted the presence or severity of depression, which indicated that physicians must continue to rely on screening instruments or clinical examination. In addition, we were unable to comment on the impact of the treatment of depression with counseling and/or antidepressants. Only two studies reported use of these depression-specific therapies, and medications used and compliance were not reported.7,8 CRS patients with untreated depression may experience poorer QOL when compared to those undergoing depression counseling or pharmacotherapy.
There are a number of potential mechanisms to explain the increased prevalence of depression in CRS. Previous studies showed a wide spectrum of systemic effects in patients with CRS, including sleep dysfunction, anxiety, and cognitive impairment.14,24,25 All of these systemic morbidities can increase the likelihood of depression, and the relationship among these systemic disorders is complex.26 In addition, patients with CRS may feel socially isolated due to sinonasal symptoms, such as impaired olfaction and/or taste, nasal obstruction, and drainage. Similar to other chronic illnesses, patients with CRS have to spend significant time with physician visits and health care activities, e.g., sinus lavage.27 This lost recreational time likely results in frustration and potential depression. Also, in other chronic illnesses, a systemic inflammatory hypothesis has been proposed, which links inflammatory cytokine levels to depression severity.26 Cancer patients are known to have elevated levels of systemic interleukin 6 and 1β that correlate with fatigue and behavioral symptoms, and similar increases in circulating proinflammatory cytokines may contribute to depression associated with other illnesses, including multiple sclerosis, rheumatic disease, asthma, and allergies.26 The systematic inflammatory hypothesis has not yet been studied in CRS.
The relationship of comorbid depression with CRS outcomes is complex. Similar to studies of comorbid depression in other chronic illnesses,4,5 most studies in CRS demonstrate that patients with depression still improve after treatment but may not achieve equivalent long-term outcomes. Put another way, patients with depression may improve to a similar relative degree, but their QOL remained below that of patients without depression. This finding certainly has implications for prognosis and patient counseling regarding long-term expectations. The potential bidirectional association of depression and CRS makes it difficult to determine why patients may fail to achieve similar long-term QOL levels as peers without depression. One could hypothesize that depression is a secondary outcome of a particularly severe phenotype of CRS or, alternatively, that ongoing depression impacts patient-reported QOL, independent of CRS severity. Regardless, more study is required to parse out these mechanisms and develop treatment recommendations.
CONCLUSION
Depression is commonly associated with CRS and likely underdiagnosed in many patients. It is associated with worse QOL and likely poorer absolute outcomes after CRS treatment. To obtain optimal posttreatment results, it is critical that we gain a better understanding of depression in CRS and develop strategies to treat this important comorbidity.
Footnotes
Supported by a grant from the Flight Attendant Medical Research Institute (ID 113042 CIA). Z.M. Soler is also supported by grants from the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, Bethesda, Maryland (R01 DC005805; R03 DC013651–01)
Z.M. Soler is a consultant for Olympus. R.J. Schlosser is supported by grants from OptiNose, Entellus, and IntersectENT, which are not associated with this manuscript, and is also a consultant for Olympus and Meda, which are not affiliated with this study. The remaining authors have no conflicts of interest pertaining to this article
Presented in part at the North American Rhinology and Allergy Conference, St Thomas, U.S. Virgin Islands, January 14–17, 2016
REFERENCES
- 1. Maurer DM. Screening for depression. Am Fam Physician 85:139–144, 2012. [PubMed] [Google Scholar]
- 2. Siu AL, US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA 315:380–387, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Witters D, Liu D, Agrawal S. Depression costs US workplaces $23 billion in absenteeism. 2015. Available online at http://www.gallup.com/poll/163619/depression-costs-workplaces-billion-absenteeism.aspx accessed March 3, 2016.
- 4. van der Zwaan GL, van Dijk SE, Adriaanse MC, et al. Diagnostic accuracy of the Patient Health Questionnaire-9 for assessment of depression in type II diabetes mellitus and/or coronary heart disease in primary care. J Affect Dis 190:68–74, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chronic Obstruct Pulmon Dis 9:1289–1306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG. and PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 151:264–269, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Brandsted R, Sindwani R. Impact of depression on disease-specific symptoms and quality of life in patients with chronic rhinosinusitis. Am J Rhinol 21:50–54, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Mace J, Michael YL, Carlson NE, et al. Effects of depression on quality of life improvement after endoscopic sinus surgery. Laryngoscope 118:528–534, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Wasan A, Fernandez E, Jamison RN, Bhattacharyya N. Association of anxiety and depression with reported disease severity in patients undergoing evaluation for chronic rhinosinusitis. Ann Otol Rhinol Laryngol 116:491–497, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: A multi-institutional prospective cohort study. Otolaryngol Head Neck Surg 142:55–63, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis GE, Yueh B, Walker E, et al. Psychiatric distress amplifies symptoms after surgery for chronic rhinosinusitis. Otolaryngol Head Neck Surg 132:189–196, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Smith TL, Mendolia-Loffredo S, Loehrl TA, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 115:2199–2205, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Sahlstrand-Johnson P, Ohlsson B, Von Buchwald C, et al. A multi-centre study on quality of life and absenteeism in patients with CRS referred for endoscopic surgery. Rhinology 49:420–428, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Tomoum MO, Klattcromwell C, DelSignore A, et al. Depression and anxiety in chronic rhinosinusitis. Int Forum Allergy Rhinol 5:674–681, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Nanayakkara JP, Igwe C, Roberts D, Hopkins C. The impact of mental health on chronic rhinosinusitis symptom scores. Eur Arch Otorhinolaryngol 270:1361–1364, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Litvack JR, Mace J, Smith TL. Role of depression in outcomes of endoscopic sinus surgery. Otolaryngol Head Neck Surg 144:446–451, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlosser RJ, Hyer JM, Smith TL, et al. Depression-specific outcomes after treatment of chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg 142:370–376, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlosser RJ, Storck K, Cortese BM, et al. Depression in chronic rhinosinusitis: A controlled cohort study. Am J Rhinol Allergy 30:128–133, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jung YG, Lee JS, Park GC. Does post-infectious olfactory loss affect mood more severely than chronic sinusitis with olfactory loss? Laryngoscope 124:2456–2460, 2014. [DOI] [PubMed] [Google Scholar]
- 20. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.). 2000. doi:10.1176/appi.books.9780890423349. [Google Scholar]
- 21. Chung JH, Lee YJ, Kang TW, et al. Altered Quality of Life and Psychological Health (SCL-90-R) in patients with chronic rhinosinusitis with nasal polyps. Ann Otol Rhinol Laryngol 124:663–670, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Katotomichelakis M, Simopoulos E, Tripsianis G, et al. Predictors of quality of life outcomes in chronic rhinosinusitis after sinus surgery. Eur Arch Otorhinolaryngol 271:733–741, 2014. [DOI] [PubMed] [Google Scholar]
- 23. O'Connor E, Whitlock EP, Gaynes B, Beil TL. Screening for depression in adults and older adults in primary care: An updated systematic review. U.S. Preventive Services Task Force Evidence Syntheses. Evidence Synthesis No. 75. AHRQ Publication No. 10–05143-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2009. [PubMed] [Google Scholar]
- 24. Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope 123:2364–2370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soler ZM, Eckert MA, Storck K, Schlosser RJ. Cognitive function in chronic rhinosinusitis: A controlled clinical study. Int Forum Allergy Rhinol 5:1010–1017, 2015. [DOI] [PubMed] [Google Scholar]
- 26. Felger JC, Lotrich FE. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 246:199–229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. Laryngoscope 125:1547–1556, 2015. [DOI] [PubMed] [Google Scholar]