Abstract
Chest drain insertion in inexperienced hands carries a significant morbidity and mortality. The royal colleges, recognising this, stipulated that chest drain insertion be included as one of the core competences for all core medical trainees. However, there is no formal training in chest drain insertion included in training programmes. Simulation training should, in theory, provide a safe and objective method to overcome the obstacles in chest drain insertion training. There have been a number of attempts to find the ideal simulator for chest drain insertion with varying success. This article describes a model which is practical and affordable in all clinical skills labs, using porcine ribs mounted on a resin cast of a human thorax, and the data about the validation of the porcine-thorax model for chest drain insertion presented.
Key Words: chest drain insertion, core medical training, porcine-resin model, simulation training, validity
Introduction
The Federation of the Royal Colleges of Physicians stipulates that chest drain insertion is one of the core competences for all core medical trainees (CMT) by specialist trainee level 2.1 The introduction of the European Working Time Directive in UK hospitals has changed the training of the junior medical doctors to a significant extent by reducing the available hours for patient contact.2 This depletes opportunities to gain experience inserting chest drains. In a recent survey among medical registrars in the West Midlands Deanery, 21% were not confident of performing a chest drain and 32% were not confident to supervise the juniors. Unfortunately, chest drain insertion continues to be a significant source of preventable morbidity and mortality as highlighted by the National Patient Safety Agency's rapid response report in 2008.3–5
Simulators can create a risk-free environment where trainees can do deliberate practice to shorten the learning curve by improving their skills as well as their confidence. Simulation is perhaps best known in the fields of aviation and the military. In particular, it has been the norm in training pilots for more four decades.6 Surgical and anaesthetic colleagues have embraced simulation much earlier than other specialties.7–11 There have been a number of attempts to find the ideal simulator for chest drain insertion. Some have used animal cadavers and rib cages, live dogs, mannequins and human cadavers; some of these are expensive and not practical in every skills lab.12–15 Interestingly, very few have been rigorously tested for their validity in training as well as assessment. This article describes a model which is practical and affordable, using porcine ribs mounted on a resin cast of a human thorax.
Successful integration of any new simulation system for training needs rigorous evaluation. There is widespread acceptance of Gallagher et al's recommendation for validation of surgical simulators.16,17 The fundamental entities of validation include:
Face validity: the extent to which the simulator resembles real life situations as judged by the trainees as well as the trainers.
Content validity: detailed examination of the simulator by experts in the field for appropriateness of the simulator for the use of simulation.
Construct validity: the ability of the simulator to distinguish the experienced from the novice.
The results for face, content and construct validity of the porcine-resin thorax model are presented here.
Development of the simulator
The model is a life-sized resin cast of the human thorax with a triangular-shaped window in the axilla to represent the ‘safety triangle’. An appropriate-sized porcine chest wall incorporating the lower five ribs is sutured into the window. This presents the operator with skin, subcutaneous tissue, muscles and rib space very similar to a human thorax. For safety and use of animal products, the skills lab was assessed and passed fit to practice by the local microbiology and infection control departments.
Materials and methods
Participants to the study were recruited via internal emails and flyers to all the trainees and physicians across all hospitals in the West Midlands Deanery. They were divided into three groups. Group 1 consisted of 50 CMTs who had performed less than five chest drain insertions (novices), group 2 consisted of 30 registrars who have completed between five and 20 procedures (intermediate), group 3 consisted of 20 senior respiratory registrars and respiratory consultants who had performed more than 20 procedures.
None of the participants had seen or used the model before the training sessions. The sessions began with a two-hour period where the participant performed a chest drain insertion without any training. Then a 10-minute video of chest drain insertion in the model was shown, followed by a demonstration of chest drain insertion in the model by the researchers. Finally one hour of further practice using the model by the participants was undertaken.
Face and content validity
All participants filled in a questionnaire after performing a chest drain insertion using the Seldinger technique on the model. The questionnaire comprised of seven questions on realism, such as anatomy, tactility, guide wire insertion, dilatation, tube insertion, securing the drain and overall realism of the model compared with an actual patient. These were answered using a seven-point Likert scale ranging from ‘absolutely not realistic’ to ‘absolutely realistic’. A further four questions were put to ‘the intermediate’ and the ‘expert’ group regarding the appropriateness of the simulator as a training tool for different levels of trainees and an assessment tool in competency assessment. The answers were rated on a seven-point Likert scale ranging from ‘strongly disagree’ to ‘strongly agree’.
Construct validity
All the participants were instructed to insert chest drains in the porcine-resin model using the Seldinger technique. The performance was videoed and anonymised by muting the sound as well as including only the doctor's hands and arms in the video. The chest drain insertion procedure was broken down into 10 steps and each step was scored by a checklist scoring method. Each step was scored on a scale 0–5.
Results
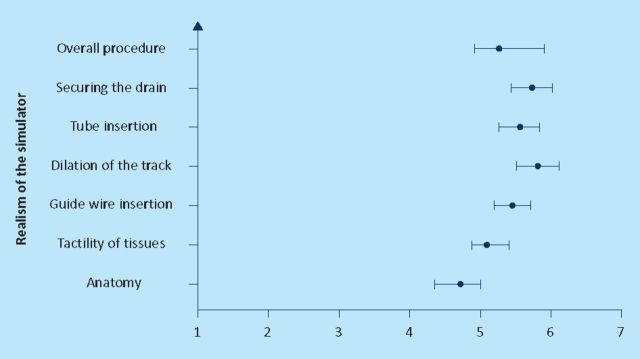
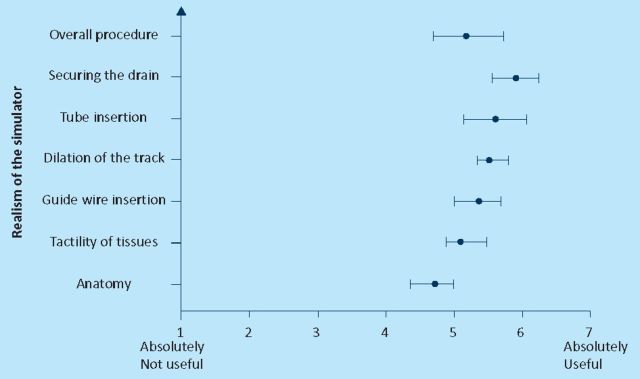
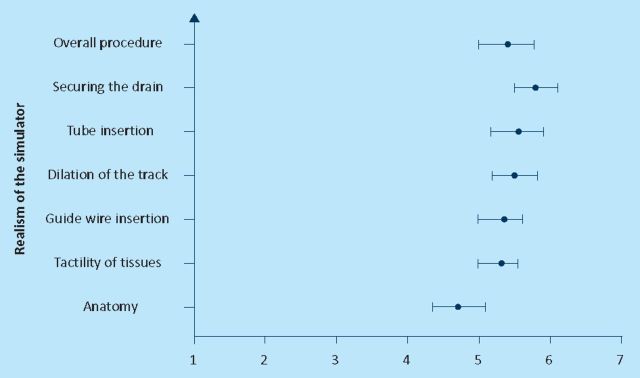
Figures 1–3 show the mean score and the 95% confidence interval for the seven questions that were asked about the realism of the simulator to all the groups. Statistically there was not any significant difference between the answers from the three groups. Lowest scores (mean 4.8) were given to the anatomy of the model and the highest was given to securing the drain (mean 6.1). Overall mean was 5.2 out of seven.
Fig 1.
Face validity: novice.
Fig 3.
Face validity: experts.
Fig 2.
Face validity: intermediate group.
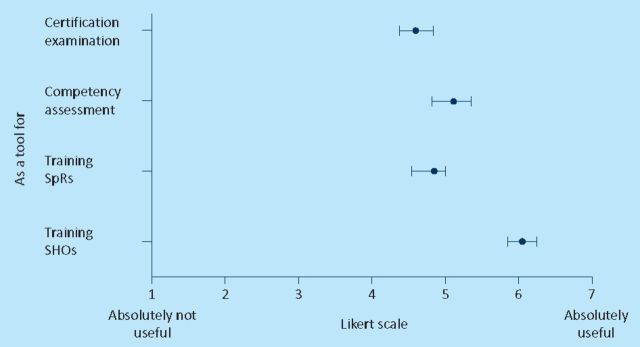
Figure 4 shows the mean score and the 95% confidence interval for questions regarding the usefulness of this simulator for training and assessment purposes. The large majority of the participants agreed that the porcine-resin model is a useful tool to train junior doctors.
Fig 4.
Content validity.
Construct validity
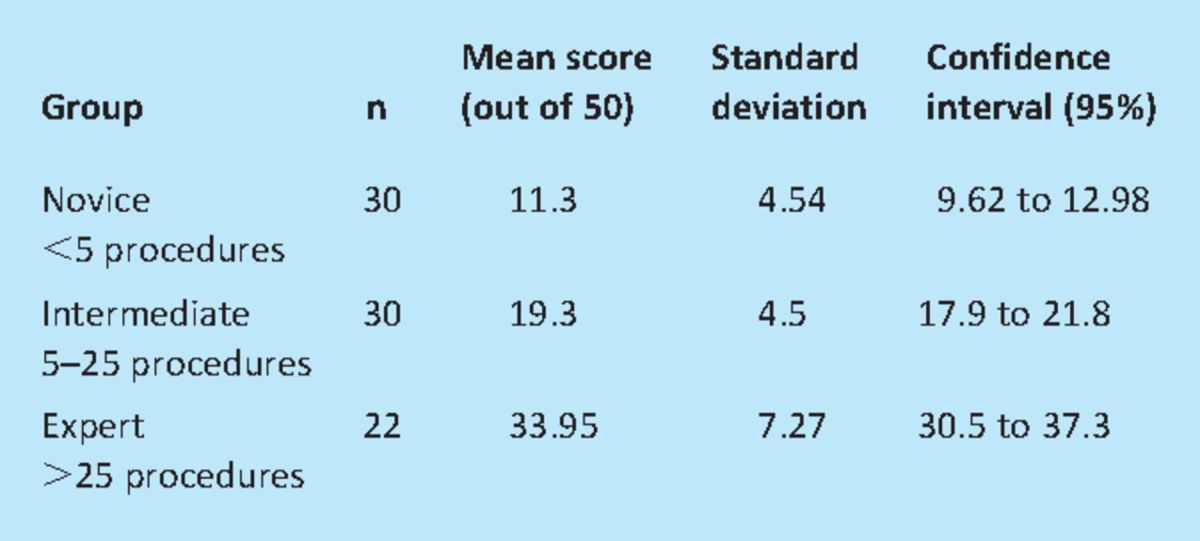
In order to obtain true ability of the model to discriminate construct validity was assessed before any training by scoring the participants using a checklist scoring system. The results are shown in Table 1 and when the scores between the groups were compared the novices scored significantly lower than the intermediate group (p<0.001) and they in turn scored significantly lower than the expert group (p<0.001).
Table 1.
Results
Discussion
The rapid increase in the number of trainee physicians and shortening the amount of time they spend in contact with the patient has imposed a huge burden on medical training, especially procedural skills. Trainees are battling with an uphill struggle to shorten the learning curve and are finding it difficult to achieve. Simulation gives them a unique platform to acquire the skills in a controlled environment without the risk of harm to patients.
Though there are a number of commercial models available, as far as can be ascertained from peer-reviewed publications, none have been validated formally as a model suitable for training and assessment of chest drain insertion. In addition, the resin-porcine thorax model is considerably cheaper at £200 and is reusable with an additional cost of just £10 per session towards the cost of fresh porcine ribs, whereas plastic models from commercial manufacturers cost anywhere between £1,000 and £2,500 with additional materials costing about £50 per session.
For validation purposes, methods widely accepted and recommended by Gallagher et al have been used for validation of surgical and endoscopy simulators.16,17 Before deciding to formally validate the use of this model, a training need analysis was completed. The survey was conducted in the West Midlands Region among specialist registrars (n=106) who are expected to insert chest drains or supervise juniors. They almost unanimously (99%) reported that they did not have any formal training in chest drain insertion and 21% admitted that they were not confidant in performing the procedure. This audit showed that only four of the 19 hospitals had a formal programme in place for chest drain insertion. This further strengthens the case to develop a validated training tool.
The answers to the face validity questionnaire demonstrated that everyone, from the novice to the expert, agrees that the overall realism of the model is good, though they were undecided about the comparable anatomy due to the slightly thicker chest wall in pigs. Selection bias also has to be acknowledged–because the participants entered the study voluntarily they might be biased towards the use of the simulator. There are also some limitations in asking the novice group to compare the model with the real procedures as they had not performed many of the latter. They can, however, appreciate the tactile difference between fresh flesh with its readily visible ribs and intercostals muscles as well as the sensation of putting a stitch through skin and subcutaneous tissues compared to plastic.
There is a possibility of social desirability effect in that the participants may have felt obliged to give a positive report about the model to please the researchers as they have put personal effort and time to teach them a new procedure. In addition, the participants may have been influenced by the novelty of the model. There does seem to be, however, an overwhelming consensus in favour of the model among all groups.
Experts were asked to assess the potential use of this model in training as well as assessment. There was an overwhelming enthusiasm among the experts to use this for training juniors in chest drain insertion. They also thought that this would be a good tool to assess competency. The porcine-resin model was able to differentiate participants with varying experience in chest drain insertion. The mean score of each group was proportionate to their experience in chest drain insertion and, in effect, it can differentiate the novice from expert.
There is an urgent need for an alternative teaching method in order to develop procedural skills in this generation of physicians. This study has demonstrated that this model is easy to develop, is cost effective and can be adopted in all clinical skills labs across the country. The challenge is to find out how much of these skills learned in the laboratory setting will be transferred to the bedside. The novice group will be followed for another year to find out if there is a correlation between the skills lab score and the actual score when performing on patients (predictive validity).
Conclusion
The face, content and construct validity for this resin-porcine model to simulate chest drain insertion training has been established. It is a portable and easily reproducible cost-effective simulator and should be included in CMT programmes in all deaneries.
References
- 1.Federation of the Royal Colleges of Physicians. The physician of tomorrow 2007London: Federation of the Royal Colleges of Physicians [Google Scholar]
- 2.Tsouroufli M, Payne H. Consultant medical trainers, Modernising Medical Careers (MMC) and the European Time Directive (EWTD): tensions and challenges in a changing medical education context. BMC Med Educ 2008; 8: 31. 10.1186/1472-6920-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collop NA, Kim SK, Sahn SA. Analysis of tube thoracostomy performed by pulmonologists at a teaching hospital. Chest 1997; 112: 709–13 10.1378/chest.112.3.709 [DOI] [PubMed] [Google Scholar]
- 4.Deneuville M. Morbidity of percutaneous tube thoracostomy in trauma patients. Eur J Cardiothoracic Surg 2002; 22: 673–8 10.1016/S1010-7940(02)00478-5 [DOI] [PubMed] [Google Scholar]
- 5.National Patient Safety Agency Rapid Response Report, NPSA/2008/RRR003.
- 6.Hudson P. Applying the lessons of high risk industries to Health care. Qual Saf Health Care 2003; 12Suppl 1i7–i12 10.1136/qhc.12.suppl_1.i7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champion HR, Gallagher AG. Surgical simulation–a ‘good idea whose time has come’. Br J Surg 2003; 90: 767–8 [DOI] [PubMed] [Google Scholar]
- 8.Glavin R.155–61Simulation in anaesthesia and acute care settings. Conference Proceedings. Euroanaesthesia 2005: Vienna, Austria
- 9.Ziv A, Wolpe PR, Small SD, Glick S. Simulation-based medical education: an ethical imperative. Acad Med 2003; 78: 783–8 10.1097/01.SIH.0000242724.08501.63 [DOI] [PubMed] [Google Scholar]
- 10.Cumin D, Merry AF, Weller JM. Standards for simulation. Anaesthesia 2008; 63: 1281–7 10.1111/j.1365-2044.2008.05787.x [DOI] [PubMed] [Google Scholar]
- 11.Sturm LP, Windsor JA, Cosman PH, et al. A systematic review of skills transfer after surgical simulation training. Ann Surg 2008; 248: 166–79 10.1097/SLA.0b013e318176bf24 [DOI] [PubMed] [Google Scholar]
- 12.Homan CS. Evaluation of an emergency–procedure teaching laboratory for the development of proficiency in tube thoracotomy. Acad Emerg Med 1994; 1: 382–7 [DOI] [PubMed] [Google Scholar]
- 13.Proana L, Jagminas L, Homan CS, Reinert S. Evaluation of a teaching laboratory using a cadaver model for tube thoracostomy. J Emerg Med 2002; 23: 89–95 10.1016/S0736-4679(02)00468-7 [DOI] [PubMed] [Google Scholar]
- 14.Berkenstadt H, Yaron M, Gregory T, et al. Evaluation of Trauma-Man™ Simulator for training in chest drain insertion. Eur J Trauma 2006; 32: 523–6 [Google Scholar]
- 15.Hutton IA. Using simulation models to teach junior doctors how to insert chest tubes: a brief and effective teaching module. Int Med J 2008; 38: 887–91 10.1111/j.1445-5994.2007.01586.x [DOI] [PubMed] [Google Scholar]
- 16.Gallagher AG, Ritter EM, Satava RM. Fundamental principles of validation, and reliability: rigorous science for the assessment of surgical education and training. Surg Endosc 2003; 17: 1525–9 10.1007/s00464-003-0035-4 [DOI] [PubMed] [Google Scholar]
- 17.Schout BMA. Validation and implementation of surgical simulators: a critical review of present, past, and future. Surg Endosc 2010; 24: 536–46 10.1007/s00464-009-0634-9 [DOI] [PMC free article] [PubMed] [Google Scholar]