Key points
Although pH1N1 is now in the post-pandemic phase, physicians should be vigilant to identify patients presenting with respiratory symptoms during the winter influenza season
Conditions associated with an increased risk include asthma, cardiac disease, immunosuppression, pregnancy and post-partum states, diabetes mellitus and obesity
Patients may be afebrile or have predominantly gastrointestinal symptoms at presentation
Reverse transcriptase polymerase chain reaction of nasopharyngeal or throat swabs is the most effective method of diagnosis, but false-negatives may occur in the presence of viral pneumonia
Patients should be retested and antiviral medication continued if there is a high clinical index of suspicion
Antiviral drugs are most effective when administered early, but may confer benefit up to seven days after the onset of symptoms
Vaccination is safe, effective and dramatically underused
Influenza viruses are a significant cause of morbidity and mortality globally, resulting in severe illness in 3–5 million people and death in up to 500,000 during epidemic years.1 These viruses are members of the orthomyxoviridae family and subclassified into influenza A, B and C, of which only influenza A has pandemic potential. Influenza pandemics occur when virus expressing a novel haemagglutinin or neuraminidase surface glycoprotein infects a population with no prior immunity. The devastating effects of this were demonstrated by the 1918 H1N1 pandemic in which approximately 20–50 million people died worldwide.
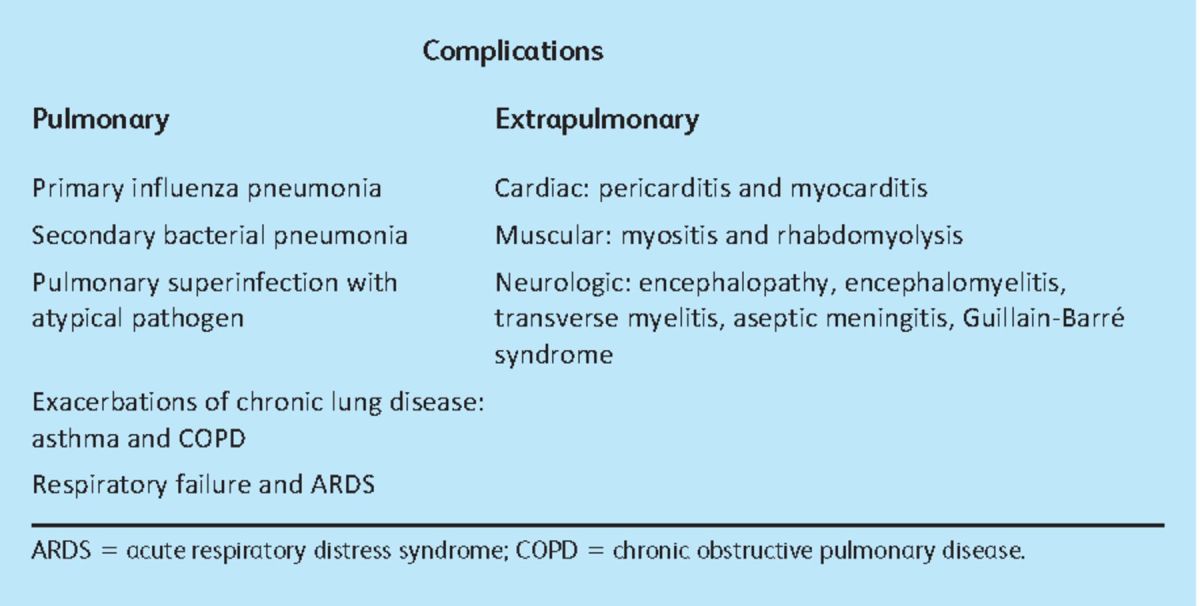
Although it is now generally considered that the 2009 H1N1 pandemic resulted in mild disease in most individuals, serious complications still occurred (Table 1).2 Moreover, a greater number of individuals in the UK became critically ill during the post-pandemic phase in the winter of 2010 than in the previous year. Consequently, physicians should remain vigilant to identify patients with influenza who develop respiratory symptoms.3
Table 1.
Complications of seasonal and pandemic influenza. Adapted from reference 2.
This article focuses on clinical aspects of the 2009 H1N1 influenza A (pH1N1) infection in adults, in particular the pulmonary complications of primary viral and secondary bacterial pneumonia.
Origins and epidemiology
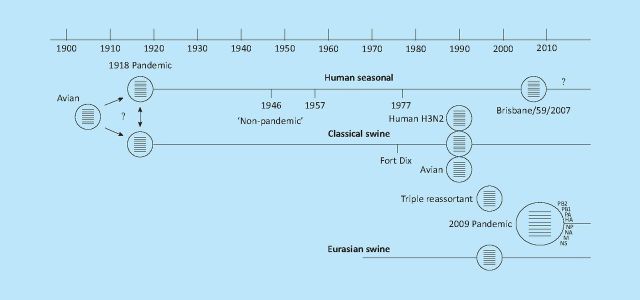
Following the emergence of the first cases of pH1N1 in Mexico in March 2009 the virus spread rapidly, achieving pandemic status within three months. The pH1N1 virus arose from the combination of two genes from a Eurasian swine virus with six genes from a ‘triple-reassortant’ North American swine virus lineage (Fig 1).4 In the US alone the pandemic accounted for an estimated 59 million illnesses, 265,000 hospitalisations and 12,000 deaths by mid-February 2010.5
Fig 1.
Origins of H1N1/09.4 The 2009 pandemic H1N1 virus resulted from the reassortment of a Eurasian avian-like H1N1 lineage that emerged in pigs around 1970 with a ‘triple reassortant’ virus that emerged in pigs in the late 1990s. The ‘triple reassortant’ contains the PB2 and PA of an avian virus, PB1 and NA of a human H3N2 and four genes from classical swine virus that had been in circulation since the 1918 H1N1 pandemic.
The pandemic virus differed from seasonal influenza in its propensity to affect young and middle-aged adults (aged 20–65) rather than the elderly. In the UK, 474 deaths were reported in the pandemic phase, one-third of which occurred in patients with minimal or no underlying health problems.6 Risk factors for severe disease included asthma, cardiac disease, immunosuppression, pregnancy and post-partum states, diabetes mellitus and obesity. Fewer than 1% of patients in the UK were admitted to hospital, but 12–15% of these required critical care, placing a huge burden on services.7
Influenza-related pneumonia was found in 40% of hospitalised patients in the US, and in Australasia 49% of the critically ill patients were diagnosed with viral pneumonia and 20% had secondary bacterial pneumonia.7
Pathogenesis
Primary influenza pneumonia and post-influenza secondary bacterial pneumonia are distinct pathologies but difficult to distinguish clinically. The pathogenesis of primary influenza pneumonia in pH1N1 infection is not yet fully understood, but the end-point is evident from autopsy studies showing diffuse alveolar damage (DAD) associated with haemorrhage and necrotising bronchiolitis.8 The mechanism by which pH1N1 induces DAD is unclear, although it is likely to be a result of the cytopathic effects of the virus in combination with an overexuberant inflammatory response.
Laennec first established the relationship between influenza infection and secondary bacterial pneumonia in 1803.9 Numerous mechanisms for increased post-influenza bacterial susceptibility have subsequently been proposed, including:
Clinical features and diagnosis
The clinical diagnosis of pandemic influenza is based on:
the presence of fever (>38oC), or
a history of fever and influenza-like illness (two of more of the symptoms of cough, sore throat, rhinorrhoea, limb or joint pain, headache, vomiting or diarrhoea), or
severe or life-threatening illness suggestive of an infectious process.
Notably, gastrointestinal symptoms (diarrhoea, vomiting and abdominal pain) are more prominent in pH1N1 relative to seasonal influenza (<10%), and up to one-third of patients may be afebrile at presentation. Most adults with pH1N1 experience mild symptoms, with 50% recovering within seven days of symptom onset and a further 25% within 10 days.7
Laboratory investigations
Laboratory diagnosis of pH1N1 is best achieved through real-time reverse transcriptase polymerase chain reaction (rt-PCR) analysis of both combined nasopharyngeal and throat swabs and nasopharyngeal aspirates,11 plus tracheobronchial aspirates in ventilated patients. There is a significant incidence of false-negative results, so repeated testing of both upper respiratory swabs and tracheobronchial aspirates is required to exclude viral infection with confidence. If there is a high index of suspicion, antiviral therapy should be continued until repeated samples are negative.12 Although PCR is the most sensitive and specific method of diagnosis, it has limited availability and nasopharyngeal aspiration can be both unpleasant and negative in the presence of influenza-related pneumonia. Other available diagnostic assays include rapid antigen and direct immunofluorescent antibody tests, but neither can distinguish between different subtypes of influenza A nor are they as sensitive and specific as PCR.
Differentiation of influenza-related and secondary bacterial pneumonias
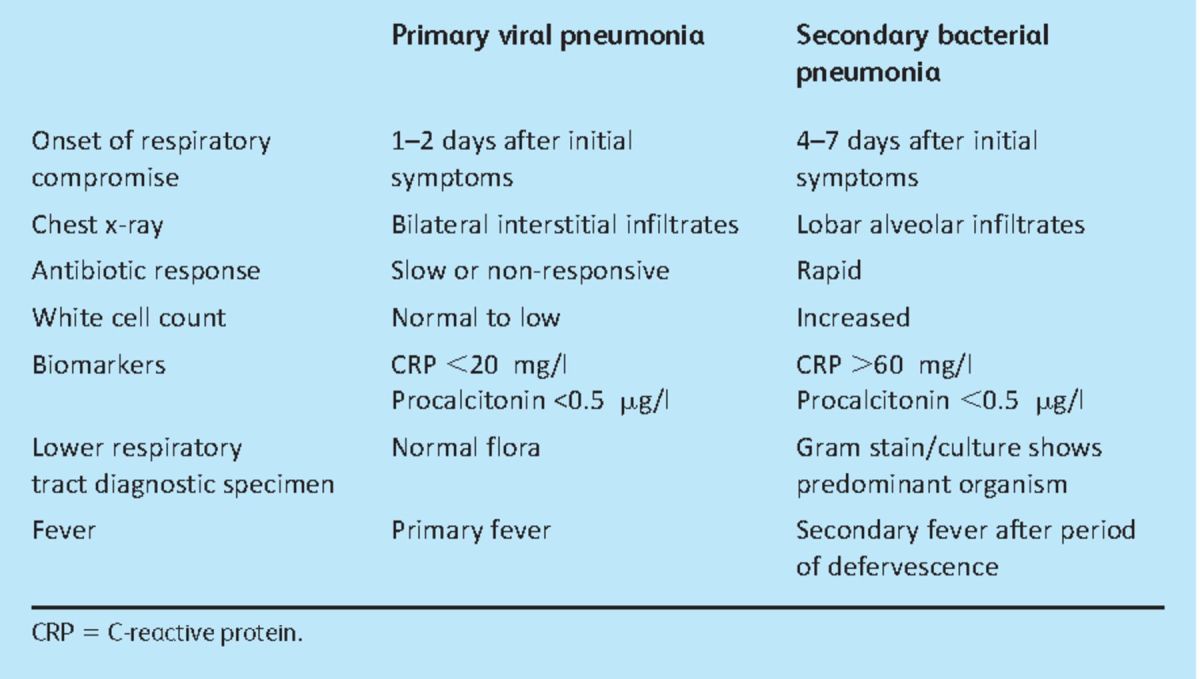
Signs suggestive of the development of influenza-related pneumonia include dyspnoea, recrudescent fever, tachypnoea, cyanosis and bilateral crepitations.13 Distinguishing between a primary viral pneumonia and secondary bacterial pneumonia can be difficult in the clinical setting. Table 2 outlines the clinical para meters that may aid differentiation.
Table 2.
Clinical parameters to aid in distinguishing primary viral pneumonia and post-influenza secondary bacterial pneumonia. Adapted from references 14 and 15.
Management
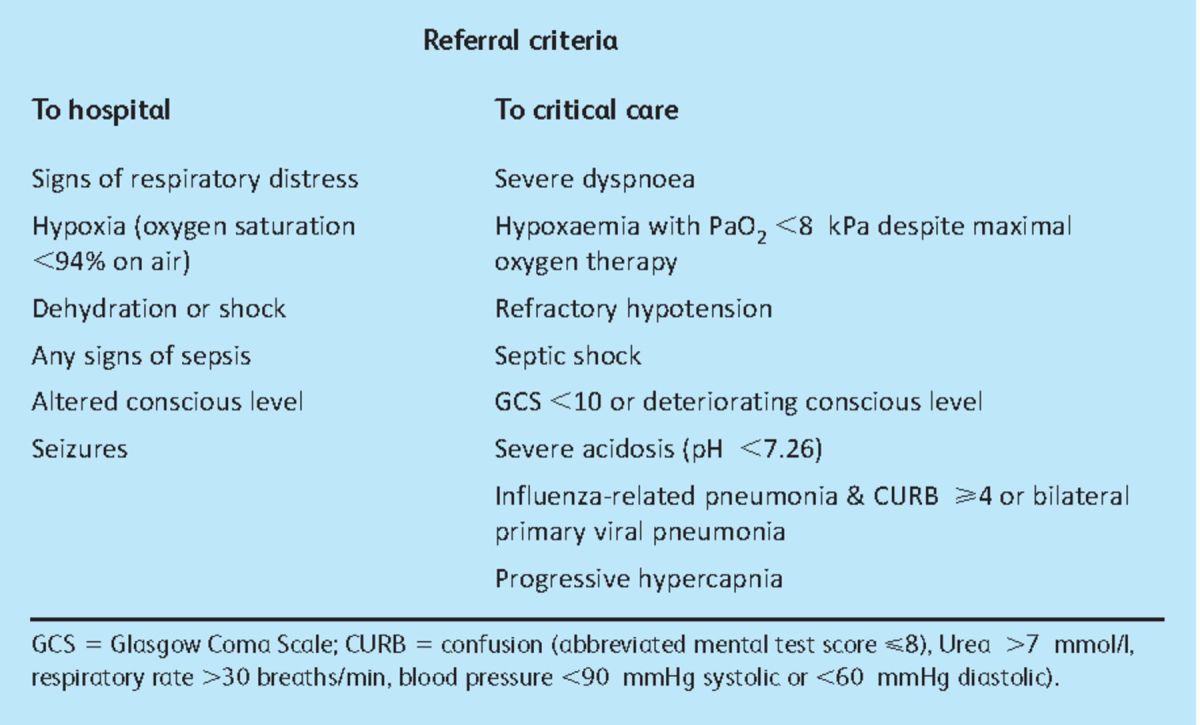
In October 2009 the Department of Health issued recommendations outlining the treatment of pH1N1 in both the primary and secondary care settings, updating previous British Thoracic Society guidelines.13 The recommendation for clinically diagnosed, uncomplicated influenza infection is prompt commencement of antiviral therapy plus symptomatic management (antipyretics, good fluid intake, smoking avoidance, rest and topical decongestants).7 The suggested criteria for hospitalisation, and subsequently for critical care admission, are outlined in Table 3. Hospitalised patients should be administered antibiotics within four hours of admission. For influenza-related pneumonia, 10 days of parenteral co-amoxiclav plus a macrolide (eg clarithromycin) should be given (for penicillin allergic patients, second-generation cephalosporins may used as an alternative), extending to 14–21 days where Staphylococcus aureus or Gram-negative enteric bacilli pneumonia is suspected or confirmed.7 The routine use of corticosteroids in influenza-associated pneumonia is not recommended,7 and its use may increase mortality.
Table 3.
The neuraminidase inhibitor, oseltamivir (75 mg orally twice daily for five days), is the drug of choice for most patients because it achieves higher systemic levels than inhaled zanamivir. Early administration of antiviral agents is associated with better prognosis16 and limits progression to pulmonary infiltrates,17 but they still confer benefit if started more than 48 hours and up to seven days after symptom onset. H1N1 is resistant to the adamantanes (eg amantadine) but oseltamivir-resistant strains have remained relatively rare, with only 45 cases recognised in the UK from 6,379 influenza-positive samples tested.18 Zanamivir (10 mg inhaled twice daily for five days) is the antiviral of choice in renal failure and for pregnant women–unless they have asthma, chronic obstructive pulmonary disease or difficulty using inhaled preparations, in which case oseltamivir should be used.7 Intravenous zanamivir is not licensed in the UK but has been used as part of a compassionate use programme for critically ill patients.
Infection control and prevention
Nosocomial influenza outbreaks were identified during the 2009 H1N1 pandemic, emphasising the need for adherence to infection control standards in hospitals.19 Hand hygiene is a critical element of infection control precautions and, in order to inactivate influenza A, should be carried out for 20–30 seconds with alcohol hand gel and 40–60 seconds hand washing (including thorough drying). Good respiratory hygiene measures (‘catch it, bin it, kill it’) should also be adhered to, not only in the ward environment but also in communal waiting areas and during patient transport. Respiratory droplets typically travel only one metre, but ideally patients should be isolated rather than cohorted, and fluid repellent surgical masks worn when working in close contact with symptomatic patients.20 Aerosol generating procedures, including the use of non-invasive ventilation, should be carried out in well-ventilated single rooms with the doors closed, wearing a gown, gloves, eye protection and a filtering face piece 3 face mask. Notably, the administration of nebulose medications and pressured humidified oxygen are not considered to represent a significant infection risk.20
Vaccination is safe and effective, and offers the best means of decreasing the number of individuals infected with influenza. In a study involving over 95,000 children and young adults in Beijing, a monovalent vaccine was 87.3% effective in preventing pH1N1 and there was no association with Guillain-Barré syndrome.21 During the winter of 2010 a trivalent vaccine that included protection against pH1N1 was available, but uptake was poor in at-risk groups and amongst healthcare workers (25% in the NHS).22 Healthcare workers in the UK accept that vaccination against hepatitis B is required to perform their role but this is not yet the case for influenza.
Summary
Influenza-related pneumonia encompasses both primary viral pneumonia and secondary bacterial pneumonia, which may be difficult to differentiate clinically. A high index of suspicion, prompt initiation of antiviral and antibiotic therapy, and appropriate escalation to secondary/critical care are key to improving outcome.
Acknowledgements
This work was funded and supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. Dr Griffiths and Dr McAuley received consultancy fees and served on advisory boards for GlaxoSmithKline (Middlesex, UK). Dr McAuley has received lecture fees from AstraZeneca (London, UK) for educational meetings.
Contributor Information
Mark H Almond, Royal Brompton and Harefield NHS Foundation Trust, Adult Intensive Care Unit, London.
Danny F McAuley, Respiratory Medicine Research Programme, Centre for Infection and Immunity, Queen's University and Regional Intensive Care Unit, Royal Victoria Hospital, Belfast.
Matt P Wise, Adult Critical Care, University Hospital of Wales, Cardiff.
Mark JD Griffiths, Royal Brompton and Harefield NHS Foundation Trust, Adult Intensive Care Unit and Unit of Critical Care, National Heart and Lung Institute, Imperial College, London.
References
- 1.World Health Organization Fact Sheet No.211. 2009. (cited 11.05.11) www.who.int/mediacentre/factsheets/fs211/en/index.html.
- 2.Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010; 384 Supple91–7 10.1097/CCM.0b013e3181c92eeb [DOI] [PubMed] [Google Scholar]
- 3.Thickett DR, Griffiths M, Perkins GD, McAuley DF. UK and Ireland Acute Lung Injury Group. Hot off the breath: the 2009 H1N1 flu pandemic may be gone but should not be forgotten. Thorax 2010; 65: 855–6 [DOI] [PubMed] [Google Scholar]
- 4.Klenk HD, Garten W, Matrosovich M. Molecular mechanisms of interspecies transmission and pathogenicity of influenza viruses: lessons from the 2009 pandemic. Bioessays 2011; 33: 180–8 10.1002/bies.201000118 [DOI] [PubMed] [Google Scholar]
- 5.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362: 1708–19 [DOI] [PubMed] [Google Scholar]
- 6.Dunning J, Openshaw PJ. Impact of the 2009 influenza pandemic. Thorax 2010; 65: 471–2 10.1136/thx.2009.133728 [DOI] [PubMed] [Google Scholar]
- 7.Department of Health Pandemic H1N1 2009 influenza: clinical management guidelines for adults and children. 2009. www.dh.gov.uk/en/Publichealth/Flu/Swinefluguidance/DH_122629.
- 8.Gill JR, Sheng ZM, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med 2010; 134: 235–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19: 571–82 10.1128/CMR.00058-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussell T, Cavanagh MM. The innate immune rheostat: influence on lung inflammatory disease and secondary bacterial pneumonia. Biochem Soc Trans 2009; 37Pt 4811–3 10.1042/BST0370811 [DOI] [PubMed] [Google Scholar]
- 11.de la Tabla VO, Masia M, Antequera P, et al. Comparison of combined nose-throat swabs with nasopharyngeal aspirates for detection of pandemic influenza A/H1N1 2009 virus by real-time reverse transcriptase PCR. J Clin Microbiol 2010; 48: 3492–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Protection Agency. Pandemic (H1N1) 2009 influenza testing in the critical care setting 2009London: HPA; www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1259152349491 [Google Scholar]
- 13.Pandemic flu: clinical management of patients with an influenza-like illness during an influenza pandemic. Provisional guidelines from the British Infection Society, British Thoracic Society, and Health Protection Agency in collaboration with the Department of Health. Thorax 2007; 62: 1–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011; 377: 1264–75 10.1016/S0140-6736(10)61459-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright PF, Kirkland KB, Modlin JF. When to consider the use of antibiotics in the treatment of 2009 H1N1 influenza-associated pneumonia. N Engl J Med 2009; 361: e112. 10.1056/NEJMopv0910749 [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 2009; 361: 1935–44 10.1056/NEJMoa0906695 [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ 2010; 341: c4779. 10.1136/bmj.c4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Protection Agency HPA Weekly National Influenza Report. Week 40. 2010. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1284475207648.
- 19.Enstone JE, Myles PR, Openshaw PJ, et al. Nosocomial pandemic (H1N1) 2009 United Kingdom, 2009–2010. Emerg Infect Dis 2011; 17: 592–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2009. Pandemic (H1N1) 2009 influenza–a summary of guidance for infection control in healthcare settings. 8/1/2010 ed.
- 21.Wu J, Xu F, Lu L, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med 2010; 363: 2416–23 10.1056/NEJMoa1006736 [DOI] [PubMed] [Google Scholar]
- 22.Department of Health. Pandemic H1N1 vaccine uptake figures for England by SHA and PCT, 2011 2011London: DH [Google Scholar]