Key points
Skeletal muscle impairment is a common and important complication of chronic obstructive pulmonary disease (COPD), associated with worse health status, functional capacity and survival
Skeletal muscle impairment occurs early in the course of the disease in association with physical inactivity, which may itself be a risk factor for COPD
Cardiovascular disease is common in patients with COPD, and COPD is common in patients with cardiovascular disease
The possibility of osteoporosis should be considered in all patients with COPD
Cognitive impairment may hamper patients' ability to learn to use inhaler devices, particularly in the context of acute exacerbations
The joint American Thoracic Society and European Respiratory Society guidelines for chronic obstructive pulmonary disease (COPD) define it as:
a preventable and treatable disease characterised by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking. Although COPD affects the lungs, it also produces significant systemic consequences.1
COPD is a disease of ageing associated with smoking, reduced physical activity, an adverse early life environment and lower socioeconomic status. As such, it shares many risk factors with other chronic conditions which frequently co-occur. Some studies have found comorbidities more common in advanced disease but this has not been a universal finding.2 The occurrence of comorbidities could represent a common susceptibility to risk factors or a systemic ‘overspill’ of lung inflammation having a direct effect on remote disease processes such as atherosclerosis.3 These two mechanisms are not mutually exclusive.
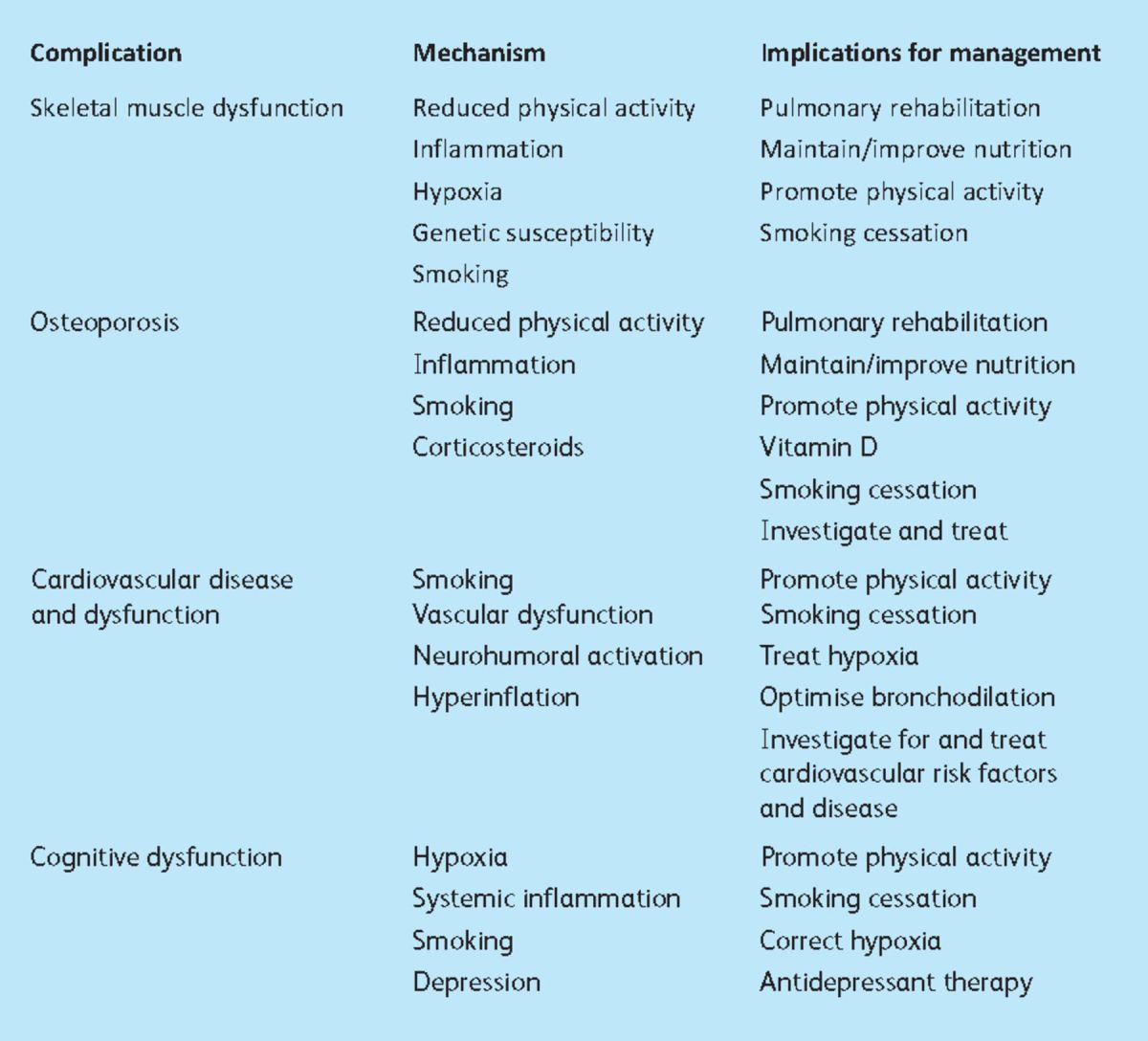
This review describes some of the important extrapulmonary features of COPD relevant to the management of patients with the disease (Table 1), rather than addressing the ontological challenge of what constitutes a comorbidity and what a complication.
Table 1.
Extrapulmonary features relevant to the management of patients with chronic obstructive pulmonary disease.
Skeletal muscle
Skeletal muscle weakness is a common complication of COPD, and is associated with reduced functional exercise capacity, impaired quality of life (Fig 1) and increased mortality.4,5 Cachexia is a well recognised feature of the end stage of many chronic diseases but it is less well appreciated that skeletal muscle weakness is a common feature of early COPD.6 This is likely to be due to reduced physical activity, which is itself a risk factor for the development of COPD.7,8
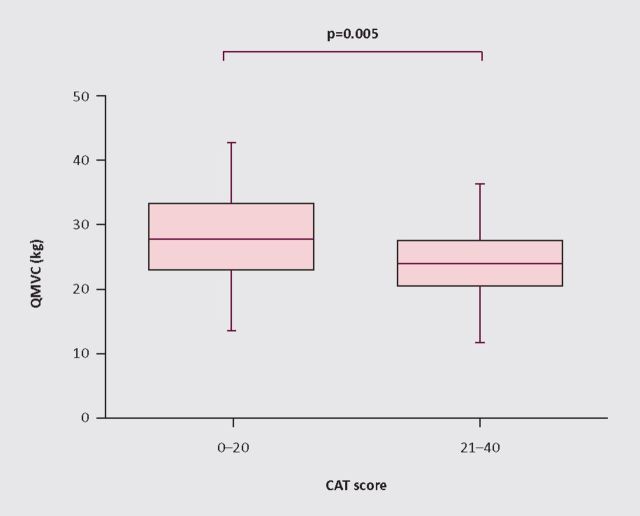
Fig 1.
The chronic obstructive pulmonary disease (COPD) assessment test (CAT) was compared with quadriceps strength in 90 outpatients with stable COPD (mean (SD) FEV1 49 (25)% predicted, age 65.7 (7.8) years, 52% male). Maximum isometric quadriceps force (QMVC)4was significantly lower in patients with worse health status (CAT score >20, n=39: QMVC 23.7 (5.6) kg vs 27.6 (6.9) kg (p=0.005), R 0.25 (p=0.02)).
The quadriceps, one of the main weight-bearing muscles, has been most extensively studied in patients with COPD. This typically displays fibre atrophy and a shift from type I, fatigue resistant, oxidative fibres towards a type II, glycolytic pattern manifest as a reduction in both strength and endurance.9 The importance of skeletal muscle is highlighted by the effect of pulmonary rehabilitation which produces dramatic increases in functional capacity and health status without changing lung function.5,10 Disuse is the main driver for muscle weakness in COPD, but other factors have been implicated including hypoxia, systemic inflammation, corticosteroids and genetic susceptibility.11 Exacerbations of COPD accentuate many of these factors and are associated with an acute loss of skeletal muscle strength which may not recover.12 Loss of fat free mass over time is associated with exacerbation rate.13 Recent data show that provision of pulmonary rehabilitation early after an exacerbation can restore function, with a number needed to treat of only four to prevent one readmission.14–16
Reduced inspiratory muscle pressures are also common in COPD. These occur largely as a consequence of hyperinflation which flattens and shortens the diaphragm, placing it at a mechanical disadvantage, rather than because of intrinsic muscle problems. Indeed diaphragm strength corrected for lung volume is normal in COPD.17
Cardiovascular disease
Cardiovascular events are common in patients with COPD and COPD is a frequent finding in patients with established cardiovascular disease. Linking mechanisms include vascular dysfunction,18 neurohumoral activation,19 systemic inflammation20,21 and hyperinflation.22 Systemic inflammation, a consistent finding in COPD, is associated with the development of atherosclerosis. An analysis of data from primary care found that a diagnosis of COPD was associated with a 10.1-fold increase in risk of myocardial infarction and a 3.4-fold risk of stroke.23 Acute exacerbations of COPD are a high risk period with raised troponin, an indication of cardiac damage, occurring frequently and associated with increased mortality.24
COPD has direct effects on cardiac function. Hyperinflation with the development of raised intrathoracic pressures reduces cardiac filling and impairs cardiac output.25 Low cardiac output leads to reduced mixed venous saturations, accentuating the effects of ventilation perfusion mismatch in damaged lung. In some patients destruction of the pulmonary vascular network causes right ventricular hypertrophy (cor pulmonale). It should be noted that fluid retention in the context of hypoxia and COPD is also driven by renal mechanisms.
It remains to be established whether the appropriate response in COPD patients is to look systematically for cardiovascular risk factors and treat them aggressively, or whether a more blanket ‘population’ approach deploying strategies known to reduce cardiovascular events (eg statins, angiotensin-converting enzyme inhibitors, beta-blockers) is justified, regardless of the presence of cardiovascular disease.
Osteoporosis
Smoking, low physical activity levels, corticosteroid treatment and systemic inflammation are risk factors for osteoporosis. Osteoporosis is highly prevalent in both men and women with COPD.26 It is associated with poor nutrition and reduced time spent outdoors. Both these factors contribute to low vitamin D levels, as noted in several cross-sectional studies of COPD.27 Vitamin D is important for bone health as well as having an impact on inflammatory processes. Several trials of supplementation in COPD are ongoing. Osteoporotic vertebral collapse is a particular issue in COPD because it reduces thoracic volume and can thus limit ventilatory capacity. Consideration of bone health should be a routine part of the assessment of patients with COPD; improving physical activity and smoking cessation are key in this area.
Cognitive and nervous system problems
Peripheral neuropathy (including increased phrenic nerve conduction time) is common in COPD and increased excitability of the diaphragm motor cortex has been reported.28,29 Muscle cramps are a frequent complaint raised by COPD patients in clinical practice, but few published data are available beyond their known association with long-acting bronchodilators.
Mild cognitive impairment related to hypoxia has been described in COPD and may particularly affect information processing and verbal memory. This can have consequences for the understanding and use of self-management plans and the introduction of new devices during acute exacerbations.30 Depression is also a common feature, thought to be mainly reactive to the limitations and discomfort of the disease, without any specific features. The recent National Institute for Health and Clinical Excellence (NICE) quality standards for COPD recommend systematic use of a tool such as the Hospital Anxiety and Depression Score as part of an annual ‘comprehensive clinical and psychosocial assessment’. Treatment for depression is conventional, with exercise, talking therapies and pharmacotherapies, as appropriate. An overlap between anxiety and breathlessness can be addressed through pulmonary rehabilitation and learning techniques for breathing control such as pursed lip breathing.
Other problems
Gastro-oesophageal reflux (GORD) is a common feature of respiratory disease, perhaps because diaphragm flattening impairs the function of the gastro-oesophageal sphincter. Interestingly, symptoms of GORD were associated with frequent exacerbations in the ECLIPSE cohort.31 Anaemia of chronic disease occurs in about 10% of patients with COPD and is associated with worse quality of life and impaired survival.32
Conclusions
Patients with COPD are likely to have a range of pathologies outside the lung, acknowledged in national and international guidelines as well as in the recently published NICE quality standards for COPD. These extrapulmonary consequences need to be considered, evaluated and treated systematically, though further data are needed to identify the best strategies to pursue for screening, investigation and treatment.
References
- 1.Celli BR. MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–46 [DOI] [PubMed] [Google Scholar]
- 2.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33: 1165–85 10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 4.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62: 115–20 10.1136/thx.2006.062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011; 66: 425–9 10.1136/thx.2010.156372 [DOI] [PubMed] [Google Scholar]
- 6.Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36: 81–8 10.1183/09031936.00104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med 2007; 175: 458–63 10.1164/rccm.200607-896OC [DOI] [PubMed] [Google Scholar]
- 8.Hopkinson NS, Polkey MI. Does physical inactivity cause chronic obstructive pulmonary disease? Clin Sci (Lond) 2010; 118: 565–72 [DOI] [PubMed] [Google Scholar]
- 9.Swallow EB, Gosker HR, Ward KA, et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol 2007; 103: 739–46 10.1152/japplphysiol.00025.2007 [DOI] [PubMed] [Google Scholar]
- 10.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 4: CD003793. [DOI] [PubMed] [Google Scholar]
- 11.Shrikrishna D, Hopkinson NS. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Med COPD Update 2009; 5: 7–13 10.1016/j.rmedu.2009.01.002 [DOI] [Google Scholar]
- 12.Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58: 752–6 10.1136/thorax.58.9.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkinson NS, Tennant RC, Dayer MJ, et al. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res 2007; 8: 25. 10.1186/1465-9921-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65: 423–8 10.1136/thx.2009.124164 [DOI] [PubMed] [Google Scholar]
- 15.Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004; 329: 1209. 10.1136/bmj.38258.662720.3A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011(10)CD005305. [DOI] [PubMed] [Google Scholar]
- 17.Similowski T, Yan S, Gauthier AP, Mackle PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 1991; 325: 917–23 10.1056/NEJM199109263251304 [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med 2009; 179: 35–40 10.1164/rccm.200804-560OC [DOI] [PubMed] [Google Scholar]
- 19.Heindl S, Lehnert M, Criee CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med 2001; 164: 597–601 [DOI] [PubMed] [Google Scholar]
- 20.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59: 574–80 10.1136/thx.2003.019588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973–9 10.1056/NEJM199704033361401 [DOI] [PubMed] [Google Scholar]
- 22.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 2010; 362: 217–27 10.1056/NEJMoa0808836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feary JR, Rodrigues LC, Smith CJ, Hubbards RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010; 65: 956–62 10.1136/thx.2009.128082 [DOI] [PubMed] [Google Scholar]
- 24.Høiseth AD, Neukamm A, Karlsson BD, et al. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011; 66: 775–81 [DOI] [PubMed] [Google Scholar]
- 25.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol 2004; 96: 1920–7 10.1152/japplphysiol.00756.2003 [DOI] [PubMed] [Google Scholar]
- 26.Ferguson GT, Calverley PM, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a Revolution in COPD Health Study. Chest 2009; 136: 1456–65 10.1378/chest.08-3016 [DOI] [PubMed] [Google Scholar]
- 27.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010; 65: 215–20 10.1136/thx.2009.120659 [DOI] [PubMed] [Google Scholar]
- 28.Hopkinson NS, Sharshar T, Ross ET, et al. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol 2004; 141: 1–12 10.1016/j.resp.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 29.Jann S, Gatti A, Crespi S, Rolo J, Biretta S. Peripheral neuropathy in chronic respiratory insufficiency. J Peripher Nerv Syst 1998; 3: 69–74 [PubMed] [Google Scholar]
- 30.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J 2010; 35: 913–22 10.1183/09031936.00125109 [DOI] [PubMed] [Google Scholar]
- 31.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–38 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 32.Boutou AK, Stanopoulos I, Pitsiou GG, et al. Anaemia of chronic disease in chronic obstructive pulmonary disease: a case-control study of cardiopulmonary exercise responses. Respiration 2011; 82: 237–45 10.1159/000326899 [DOI] [PubMed] [Google Scholar]