Abstract
Breathlessness is a common symptom in respiratory, cardiovascular and malignant disease. It reduces exercise tolerance and mobility, and is an important determinant of quality of life. The multifactorial nature of the symptom often presents difficulties in understanding why individual patients are breathless, and how breathlessness should best be palliated, especially in advanced disease. However, insights into the neurophysiological factors underlying the symptom can be gained by considering the balance between the load on, and capacity of, the respiratory muscles and increased neural respiratory drive, reflecting increased respiratory effort. Mismatch between efferent neural respiratory drive and afferent feedback, reflecting the degree of neuromechanical dissociation, is also important. This paper describes mechanisms by which ventilatory load, capacity and drive may be affected by disease, and how these can be measured physiologically. The schema presented also provides a framework for understanding the mechanisms by which interventions that relieve breathlessness may have their effect.
Key Words: cardiorespiratory disease, dyspnoea, exercise tolerance, malignancy, neuromuscular disease, physiology, respiratory muscles
Breathlessness
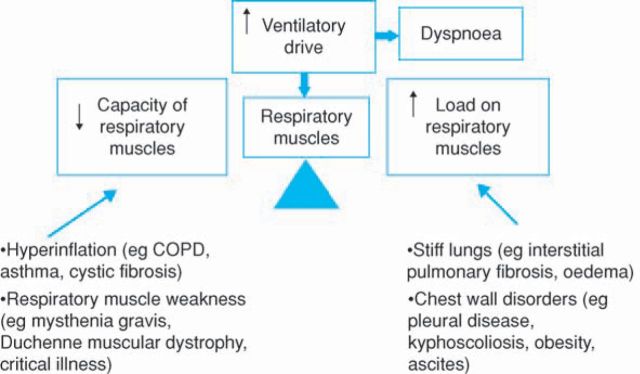
Breathlessness is common in patients with cardiorespiratory disease. Large surveys show that 70% of patients with chronic obstructive pulmonary disease (COPD) are breathless on climbing a single flight of stairs.1 In our own studies the vast majority of COPD patients stop walking because of intolerable breathlessness. Interestingly, such patients are as likely to stop cycle exercise because of leg fatigue and the important interactions between breathlessness and peripheral muscle deconditioning will be discussed later.2 Although much work on breathlessness and exercise limitation has been undertaken in patients with COPD the underlying physiological model is likely to be applicable to most breathless patients, whatever the aetiology. The simple scheme portrayed in Fig 1 is helpful. The respiratory muscles constitute the ventilatory pump responsible for achieving gas exchange. Diseases that increase the load on the respiratory muscle pump, or decrease its capacity, or both, lead to breathlessness and, eventually, ventilatory failure. The load-capacity balance is therefore crucial. The pathophysiological changes in COPD, for example, simultaneously increase the load on, and reduce the capacity of, the respiratory muscle pump. Increased airways resistance imposes a load on the respiratory muscles, as does hyperinflation (the chest is more difficult to expand at higher lung volumes), and incomplete emptying of the lung at the termination of expiration results in a positive end-expiratory pressure (intrinsic PEEP) which adds a threshold inspiratory load on the inspiratory muscles at the onset of the next breath. Clearly anything that drives an increase in ventilation (hypoxia, hypercapnia, lactataemia during exercise) will increase ventilatory load. At the same time as increasing the load on the respiratory muscles COPD reduces their capacity. Hyperinflation, a cardinal feature of moderate and severe COPD, shortens the inspiratory muscles thereby reducing their ability to generate tension. Hyperinflation also alters the geometry of the respiratory muscles, impairing their ability to bring about volume change of the thorax and therefore the lungs.
Fig 1.
A simple schematic representation of the relationship between ventilatory drive, the load on the respiratory muscles, their capacity and dyspnoea. Many cardiorespiratory disorders increase load and/or reduce capacity, which increases ventilatory drive and leads to breathlessness. COPD = chronic obstructive pulmonary disease.
Ventilatory load
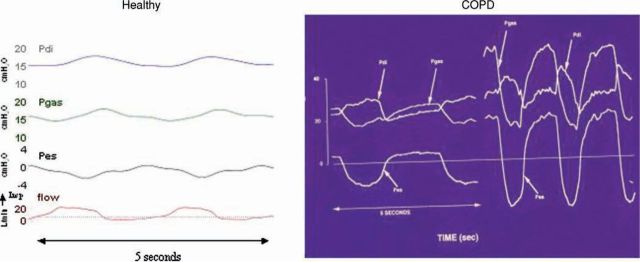
The load on the respiratory system can be quantified in numerous ways, one of which is by measuring the pressures that the respiratory muscles are required to generate to achieve ventilation. Pressure can be measured by passing balloon catheters into the oesophagus, to measure oesophageal pressure which is a close reflection of pleural pressure, and into the stomach to measure gastric pressure which reflects abdominal pressure, and the pressure gradient across the diaphragm, transdiaphragmatic pressure, which reflect the specific activity of this muscle. Figure 2 shows the changes in respiratory pressures during quiet breathing in a healthy individual. During inspiration there is a small (3 to 4 cmH2O) fall in oesophageal pressure, gastric pressure rises, and reflecting the activity of the diaphragm transdiaphragmatic pressure (Pdi) increases. Figure 2 also shows the pressure changes in a typical patient with moderately severe COPD. Note that at the onset of inspiration during quiet breathing (left COPD trace) the oesophageal pressure is positive. This represents intrinsic PEEP and inspiratory flow can only commence when this pressure has been reduced to sub-atmospheric by the contraction of the inspiratory muscles. In this COPD patient the oesophageal pressure swing during quiet breathing is about 20 cmH20. The right COPD trace shows the pressure changes during exercise, which are hugely increased. The areas under these pressure curves can be calculated, giving the pressure-time product and provide an accurate method to quantify the load on the respiratory system at rest and with exercise, in health and disease.
Fig 2.
Respiratory pressure changes during quiet breathing in a healthy individual (left), and during quiet breathing and exercise in a patient with chronic obstructive pulmonary disease (COPD) (right). Pes = oesophageal pressure; Pgas = gastric pressure; Pdi = transdiaphragmatic pressure (Pgas – Pes).
Ventilatory capacity
Turning to assessment of the capacity of the respiratory muscle pump, there are numerous measures available. The measurement most often made to assess the strength of the inspiratory muscles is maximum inspiratory mouth pressure, when making inspiratory efforts against an obstructed mouth piece (PImax). This manoeuvre is often not well performed by patients and alternative methods of measuring maximum inspiratory pressures have been developed. Most patients are familiar with the sniff manoeuvre, and perform it well. Sniff oesophageal pressure, and sniff nasal pressure can accurately assess the strength of the inspiratory muscles, and sniff transdiaphragmatic pressure specifically reflects diaphragm strength. These are useful techniques for documenting the reduced respiratory muscle capacity of patients, for example those with neuromuscular diseases, and as weakness progresses patients become breathless and develop ventilatory failure.3 For patients unable to make adequate voluntary efforts the strength of the diaphragm can be assessed by stimulating the phrenic nerves and measuring twitch transdiaphragmatic pressures.
Ventilatory load-capacity balance
A wide range of cardiorespiratory disorders increase the load on, and/or reduce the capacity of, the respiratory muscle pump, leading to breathlessness. The load is increased by airways obstruction and hyperinflation in COPD, asthma, and cystic fibrosis (CF). It is increased if the lungs are stiff (lung fibrosis or pulmonary oedema). Chest wall disorders (for example, pleural disease, kyphoscoliosis, obesity, ascites) increase respiratory load. On the other side of the balance, the capacity of the respiratory muscles is reduced by hyperinflation (COPD, asthma, CF), and by respiratory muscle weakness, as in myasthenia gravis, muscular dystrophy or critical illness. Techniques are available for measuring both the load on the respiratory muscle pump and its capacity although in practice these measurements are complex and can be difficult to apply. While it is clear that an increase in the load:capacity ratio leads to breathlessness it is also clear that an increase in the ratio causes an increase in ventilatory drive (neural respiratory drive). Indeed, a high quality measure of neural respiratory drive would provide an accurate index of the load:capacity ratio, and such a measure would help our understanding of breathlessness.
Neural respiratory drive
It is not currently possible to accurately quantify neural respiratory drive at its origin within the central nervous system. An alternative approach is to measure neural respiratory output in terms of the activation of the respiratory muscles. This is not possible for all of the relevant muscles but accurate measurements can be made for some and, of great importance, it is possible to quantify the diaphragm electromyogram (EMG). The diaphragm is the principal muscle of inspiration, responsible for 70–80% of ventilation in normal subjects. Respiratory physiologists have sought to quantify the diaphragm EMG for many years and recent advances in the technology allow accurate measurements to be made. In severe COPD the increased load on the diaphragm, and its reduced capacity, combine to cause a very large increase in neural respiratory drive to the muscle. The diaphragm EMG is a sensitive and accurate measure of disturbances in the load:capacity ratio, and this measure can be made across a wide range of cardiorespiratory diseases. The diaphragm EMG is increased (proportional to severity of disease) in, for example, asthma and obesity. As expected, there is a strong relationship between neural respiratory drive (diaphragm EMG) and the sensation of breathlessness.
Neuromechanical dissociation
A crucial factor in the perception of breathlessness is the match between input into, and output from, the central nervous system; the match between afferent feedback and efferent neural respiratory drive. Mismatch between afferent feedback and efferent neural respiratory drive is referred to as neuromechanical dissociation. The greater the degree of neuromechanical dissociation, the greater the intensity of breathlessness. A wide variety of cardiorespiratory diseases will disturb the load-capacity balance of the respiratory muscle pump and cause an increase in neural respiratory drive, and are characterised by neuromechanical dissociation. With extensive pleural thickening, for example, the achievement of adequate ventilation requires disproportionately high drive, indicative of neuromechanical dissociation, and such patients have severe breathlessness.
Neuromechanical dissociation is particularly important in COPD. The link between neural respiratory drive and ventilation is impaired. Hyperinflation is a major cause of neuromechanical dissociation, and progressive hyperinflation during exercise results in neural drive being progressively less effective in achieving ventilation. Thus, during exercise ventilation in patients with severe COPD rises relatively little whereas neural respiratory drive increases steeply.5 These changes also mean that when measuring respiratory drive the measures become progressively less accurate the more downstream they are. For example, the measurement of ventilation or pressures will less accurately reflect neural drive than the measurement of EMG.
Skeletal muscle dysfunction
In patients with cardiorespiratory disease exercise is limited by breathlessness and therefore activity levels are low. This reduced activity leads to disuse atrophy of skeletal muscle, particularly of the legs. In patients with COPD, for example, quadriceps muscle strength is reduced by 20–30%.6,7 In many disorders inflammation is an important part of the disease process, and it is thought that inflammatory mediators may contribute to the skeletal muscle dysfunction commonly observed. However, in COPD, whereas the quadriceps muscle is weak, the contractility of the adductor pollicis muscle of the hand, the abdominal muscles, and the diaphragm are not impaired suggesting that the dominant problem is disuse atrophy and weakness secondary to breathlessness.6 Muscle biopsy samples show a reduction in oxidative enzymes and type 1 fibres. Similar changes are seen in many severe chronic cardiorespiratory diseases. As a consequence of these changes in skeletal muscle, during exercise there is greater reliance on anaerobic metabolism, early and increased lactate production, increased CO2 production, and therefore higher levels of ventilation are required for a given level of exercise. The atrophy of leg muscles therefore increases ventilation and breathlessness. The patients are locked into a downward spiral of disability. Breathlessness leads to inactivity, which leads to muscle deconditioning, which itself drives breathlessness, and further reductions in activity, and so on. There is a separate, but related, vicious cycle as a result of the leg muscle dysfunction. Not only are the quadriceps weak and deconditioned, but they are also more fatiguable.8 For some activities leg fatigue can be a limiting factor. As already noted, although patients with COPD are limited by breathlessness when undertaking walking tests they are equally likely to be limited by leg fatigue when doing tests that involve cycling. While relatively few patients with COPD will cycle during daily activities most will need to walk up hills or stairs. Limitation during these activities, because of leg fatigue, is likely to contribute to exercise avoidance and therefore further deconditioning of skeletal muscle.
Rehabilitation
Within the context of the downward spiral of disability in patients with COPD, and other cardiorespiratory diseases, the powerful positive impact of rehabilitation is important. Pulmonary rehabilitation is highly effective, supported by the National Institute for Health and Clinical Excellence (NICE), but unfortunately unavailable to the majority of COPD patients. The evidence base for pulmonary rehabilitation is strong and randomised controlled trials have demonstrated increased exercise capacity and improved quality of life.9,10 Physiologically-based studies have demonstrated increased leg muscle strength, an increase in oxidative enzymes in quadriceps muscle, reduced lactate production and ventilation for a given workload, and reduced breathlessness. In short, rehabilitation programmes are able to halt and reverse the downward spiral of disability. That they are effective is not surprising given our understanding of the pathophysiology of the diseases, skeletal muscle biology, and the aetiology of breathlessness. Increased application of exercise-based rehabilitation programmes, to much larger patient populations, represents a considerable opportunity to enhance health. The benefits of exercise extend well beyond cardiorespiratory diseases and are central to the management of obesity, and it is an interesting observation that exercise in patients with breast cancer improves physical functioning.11,12
Breathlessness: management strategies
Physiological abnormalities are an essential and important aspect of any scheme that seeks to describe breathlessness. However, non-physiological aspects are also of importance. Psychological and emotional characteristics of individuals will have a big influence on the sensation of breathlessness as they will influence the intensity with which dyspnoea is experienced and also the quality of the sensation. Similarly, dyspnoea intensity and quality will influence exercise tolerance and subsequently the capacity of individuals to perform daily activities, as well as social functioning and emotional well being.
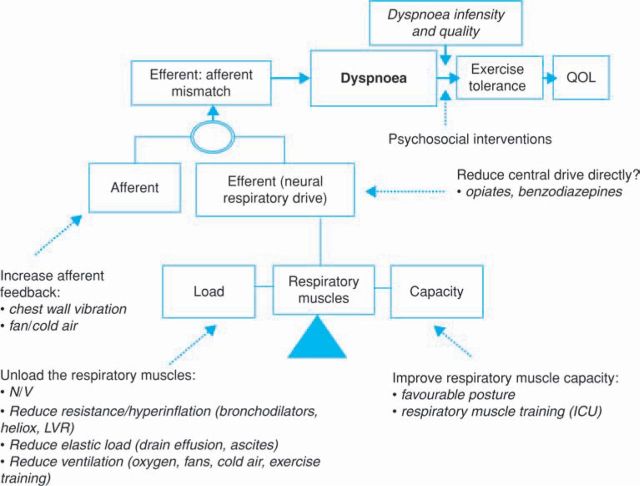
A schematic representation of breathlessness and the areas where therapies may have impact is described in Fig 3. The respiratory muscles have a central role and the load-capacity balance is crucial. There are many therapeutic possibilities to reduce the load on the respiratory muscle pump and thereby improve the load-capacity balance, and such improvements will reduce neural respiratory drive and breathlessness. For example, non-invasive ventilation (NIV) can be successful in acute exacerbations of COPD and, when effectively applied, reduces breathlessness.13 NIV can also be used to reduce the breathlessness that would otherwise limit exercise, and has been used to enable patients to achieve higher levels of exercise during training programmes. Reducing airways resistance and associated hyperinflation is a key therapeutic goal in COPD, asthma, and CF, and when successful will unload the respiratory muscles. Bronchodilators, Heliox and lung volume reduction (achieved bronchoscopically or by surgery) can all be effective. When pleural effusions are drained, ascites tapped, or pulmonary oedema treated, there is a reduction in the elastic load on the respiratory system with a consequent reduction in breathlessness. Since breathlessness is related to neural respiratory drive, any intervention that reduces ventilation will reduce breathlessness. Oxygen may therefore be helpful by this mechanism, as may fans and cold air blown on the face.
Fig 3.
A schematic representation of breathlessness mechanisms and putative mechanisms of action of therapeutic interventions. ICU = intensive care unit; LVR = lung volume reduction; NIV = non-invasive ventilation; QOL = quality of life. Adapted with permission from European Respiratory Society Journals Ltd.
Since breathlessness is closely linked to neural respiratory drive, any reduction of neural drive will reduce dyspnoea. Opiates and related drugs, often used for the palliation of breathlessness, can act in this way, although there may also be a central action serving to alter the perception of breathlessness. Clearly, the benefit of reducing neural drive has to be balanced against the effect of reducing ventilation. Many of the interventions that improve the load-capacity balance also serve to reduce the efferent-afferent mismatch. In addition, increasing afferent feedback would also be expected to reduce the sensation of dyspnoea. It is likely that the beneficial effect of chest wall vibration, reported in some studies, is achieved in this way.14
Conclusion
Understanding the physiology of the respiratory muscles, in health and disease, is crucial to understanding the mechanisms of breathlessness. The balance of the load on the respiratory muscles and their capacity is of central importance. An increase in the load:capacity ratio causes breathlessness. The most effective therapies to reduce breathlessness are those that decrease the load on the respiratory muscles, increase their capacity, or both, thereby returning the ratio towards normal. The key link between an increase in the load:capacity ratio and breathlessness is the increase in neural respiratory drive. Most interventions that reduce breathlessness will therefore also reduce neural respiratory drive. Recent advances in physiological techniques that allow us to measure neural respiratory drive (in terms of respiratory muscle EMG activity) have increased our understanding of breathlessness, and will continue to do so. Furthermore, the measurement of neural respiratory drive, because it provides an index of the load:capacity ratio, will allow us to accurately assess the impact of a wide range of therapies that affect cardiorespiratory function.
References
- 1.Rennard S, Decramer M, Calverley PM. et al Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J 2002;20:799–805. 10.1183/09031936.02.03242002 [DOI] [PubMed] [Google Scholar]
- 2.Man WD, Soliman MG, Gearing J. et al Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:562–7. 10.1164/rccm.200302-162OC [DOI] [PubMed] [Google Scholar]
- 3.Lyall RA, Donaldson N, Polkey MI, Leigh PN, Moxham J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001;124:2000–13. 10.1093/brain/124.10.2000 [DOI] [PubMed] [Google Scholar]
- 4.Polkey MI, Kyroussis D, Hamnegard CH. et al Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;154:1310–7. [DOI] [PubMed] [Google Scholar]
- 5.Sinderby C, Spahija J, Beck J. et al Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1637–41. [DOI] [PubMed] [Google Scholar]
- 6.Man WD, Soliman MG, Nikoletou D. et al Non-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax 2003;58:665–9. 10.1136/thorax.58.8.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man WD, Hopkinson NS, Harraf F. et al Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax 2005;60:718–22. 10.1136/thx.2005.040709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swallow EB, Gosker HR, Ward KA. et al A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol 2007;103:739–46. 10.1152/japplphysiol.00025.2007 [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society. Pulmonary rehabilitation Am J Respir Crit Care Med 1999;159:1666–82. [DOI] [PubMed] [Google Scholar]
- 10.Nici L, Donner C, Wouters E. et al American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390–413. 10.1164/rccm.200508-1211ST [DOI] [PubMed] [Google Scholar]
- 11.McNeely ML, Campbell KL, Rowe BH. et al Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ 2006;175:34–41. 10.1503/cmaj.051073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutrie N, Campbell AM, Whyte F. et al Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ 2007;334:517. 10.1136/bmj.39094.648553.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000;355:1931–5. 10.1016/S0140-6736(00)02323-0 [DOI] [PubMed] [Google Scholar]
- 14.Sibuya M, Yamada M, Kanamaru A. et al Effect of chest wall vibration on dyspnea in patients with chronic respiratory disease. Am J Respir Crit Care Med 1994;149:1235–40. [DOI] [PubMed] [Google Scholar]
- 15.Watson AC, Hughes PD, Harris M. et al Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 2001;29:1325–31. 10.1097/00003246-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 16.Luo YM, Hart N, Mustfa N. et al Reproducibility of twitch and sniff transdiaphragmatic pressures. Respir Physiol Neurobiol 2002;132:301–6. 10.1016/S1569-9048(02)00115-5 [DOI] [PubMed] [Google Scholar]
- 17.Harris ML, Moxham J. Measuring respiratory and limb muscle strength using magnetic stimulation. Br J Intensive Care 1998;8:21–8. [Google Scholar]
- 18.Luo YM, Hart N, Mustfa N. et al Effect of diaphragm fatigue on neural respiratory drive. J Appl Physiol 2001;90:1691–9. [DOI] [PubMed] [Google Scholar]
- 19.Jolley CJ, Rafferty GF, Steier JS. et al Measurement of the diaphragm electromyogram in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:A256. [Google Scholar]