Prescribing is a complex task requiring:
diagnostic skills
knowledge of medicines
an understanding of the principles of clinical pharmacology
communication skills
appreciation of risk and uncertainty.
The accumulation of clinical trials’ data on modern therapies might have been expected to provide sufficient evidence to support most clinical decisions. In fact, clinicians prescribe in varied circumstances, often in the absence of evidence, and rational prescribing decisions must be based on knowledge interpreted in the light of many other factors.
Rational prescribing
Rational prescribers should attempt to:
maximise clinical effectiveness
minimise harms
avoid wasting scarce healthcare resources
respect patient choice.
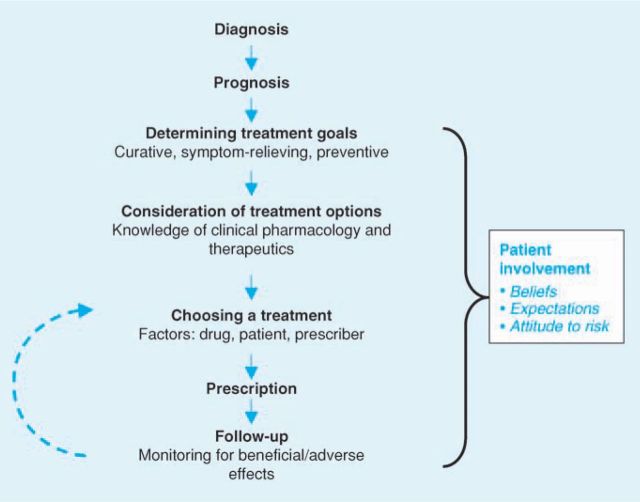
Rational prescribing normally follows a logical sequence from diagnosis to follow-up (Fig 1).
Fig 1.
The process of rational prescribing.
Diagnosis
Prescribing decisions should be based on the primary diagnosis and relevant secondary diagnoses. Ideally, these should have been made or confirmed by the prescriber who will take responsibility for the effects of treatment. Appreciating that diagnoses are made with varying degrees of uncertainty is important when assessing the benefit-to-harm balance of treatment. For instance, antibiotics are often prescribed on the basis of presumed antibacterial sensitivity with the expectation of significant benefit. However, this can expose the recipient to harm without the prospect of cure.
Prognosis
The prognoses of the primary and secondary diagnoses will affect rational treatment choices. A secondary diagnosis with a poor prognosis, such as lung cancer, will severely limit the benefits of treating a primary one, such as hypercholesterolaemia. On the other hand, the excellent prognosis of influenza in a healthy adult limits the potential benefits of antiviral therapy.
Goals of therapy
Goals of therapy may include:
curing a disease (eg cancer, infection)
relieving symptoms without affecting the underlying condition (eg headache, diarrhoea)
combining two outcomes (eg inflammatory bowel disease and arthritis)
long-term prevention (eg hypertension, osteoporosis)
replacing deficiencies (eg hypothyroidism), and occasionally
therapeutic trials to aid diagnosis.
Treatment selection
Prescribers are commonly faced with more than one choice of treatment, including non-pharmacological therapies or no treatment. For example, the management of arthritis might include reassurance, simple analgesia, physiotherapy, non-steroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, intra-articular steroids or surgery.
Monitoring
Each prescription constitutes an experiment the outcome of which is never certain. It is therefore important to monitor the effects of treatment, re-evaluate the benefit-harm balance and, if indicated, withdraw the drug or change the dose. The most appropriate end-point will be objective assessment of the clinical outcome (eg recovery from pneumonia), but assessment may be subjective (eg pain relief, improved quality of life). Patient satisfaction is also important. Sometimes the outcome is difficult to measure (eg management of epilepsy) or requires long-term follow-up (eg preservation of health in HIV infection). In such cases, validated surrogate markers (eg serum anticonvulsant concentration, CD4 cell count) may guide therapy. Adverse events can also be monitored in different ways.
Partnership with patients
Patients make important contributions to rational prescribing decisions. Their beliefs and expectations affect the goals of therapy and help in judging the acceptable benefit-harm balance when selecting treatments. They will often play a key role in monitoring treatment, not least by providing early warning of adverse events. Patients involved in clear communication with prescribers concerning reasons for drug selection, goals, duration of treatment and potential adverse effects have improved compliance, more confidence in prescribers and greater satisfaction with healthcare services. Thus, whenever possible, patients should be fully informed about their medicines (Table 1).
Table 1.
What patients need to know about their medicines.

Drug and dose selection
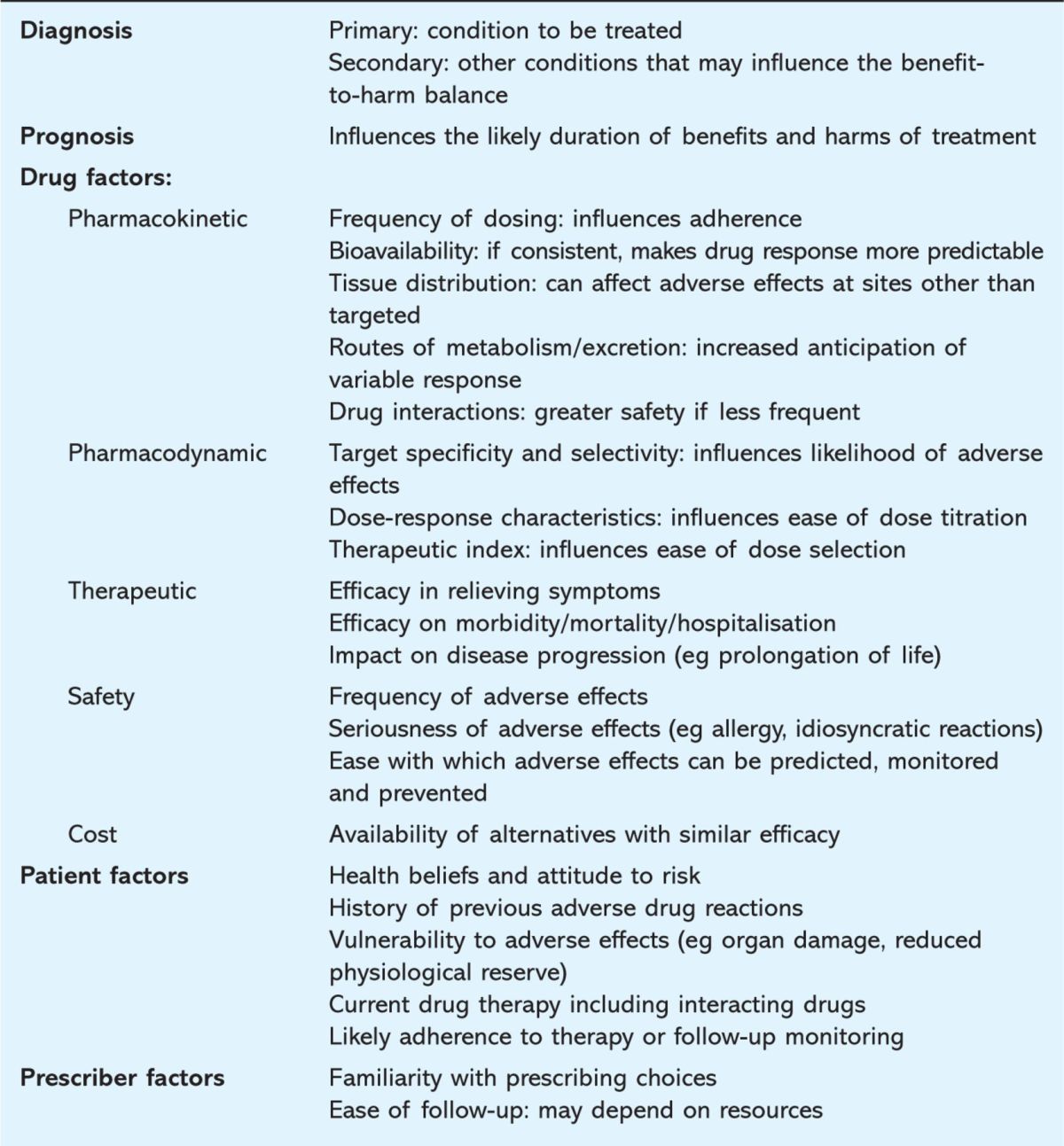
Having considered diagnosis, prognosis and goals of therapy, prescribers often select from several pharmacological options. The best choice should maximise the benefit-harm balance based on drug and patient factors, taking into account restrictions based on availability and costs (Table 2).
Table 2.
Factors that influence rational drug and dosage selection.
Drug factors influencing drug selection
Pharmacokinetics
Drugs in the same class (or different formulations of the same drug) may have different bioavailability, dose-concentration curves and half-lives. These factors will determine the dosing schedule. Once-daily dosing is convenient and encourages adherence. Pharmacokinetic characteristics may also influence interindividual variability in dosage requirements. For example, some drugs:
differ with respect to their specificity for the target organ
reach tissues (eg the brain) to cause adverse effects
are metabolised in the liver or excreted – important in patients with hepatic or renal impairment
are more likely to cause drug interactions by cytochrome P450 inhibition (eg simvastatin versus pravastatin).
Pharmacodynamics
A drug with a low therapeutic index (the ratio between the dose required to cause adverse effects and that required for efficacy) is less favourable if alternatives exist. Similarly, the steepness of the dose-response curve will influence the ease with which the dose can be optimally titrated. Selectivity for a receptor subtype may be relevant when choosing drugs that avoid predictable adverse effects. Some drugs require more complex monitoring, which can affect costs and patient time (eg warfarin versus aspirin).
Therapeutic impact and safety
A drug may be more efficacious in relieving symptoms, improving surrogate markers or preventing clinical events (eg morbidity, mortality, hospitalisation) or have fewer and less serious adverse effects (eg carbamazepine v phenytoin). Large randomised controlled trials (RCTs) are considered the optimal sources of evidence, but extrapolating the results to prescribing decisions in the real world requires caution. RCTs usually recruit highly selected participants (eg based on age or disease severity) without comorbidities or receiving interacting drugs. Such additional factors can influence efficacy or adverse outcomes, potentially reducing the former and enhancing the latter, thus limiting the external validity of RCTs.
Costs
All healthcare systems have limited resources. The rapidly increasing cost of medicines forces all prescribers to consider cost-effectiveness as a factor in drug selection. This is taken into account when devising local formularies and in the decisions of the National Institute for Health and Clinical Excellence. Perhaps the most obvious example of cost-effective prescribing is selecting a generic rather than a branded drug from the same class. However, cost may be outweighed by other factors, notably significant differences in efficacy or safety. (See accompanying article on pharmacoeconomics.)
Patient factors influencing drug selection
Previous adverse drug reactions
Knowledge of previous adverse reactions will affect drug or dose selection but depends on taking a careful drug history. This is particularly important in the case of allergic reactions (eg beta-lactam antibiotics).
Vulnerability to adverse effects
Some patients will have organ damage that may affect drug choices. For instance, a beta-blocker for angina may be undesirable in patients with peripheral vascular disease or asthma but attractive in those with heart failure. Reduced physiological reserve increases the vulnerability of elderly patients to the adverse effects of many drugs (eg anticholinergics, central nervous system depressants, vasoactive drugs) and necessitates dosage reductions.
Current drug therapy
Any current drug therapy may affect drug or dosage selection, mainly because of potential drug interactions. For example, the dose of simvastatin should not be increased beyond 20 mg nocte in patients taking amiodarone or verapamil because of the increased risk of muscle toxicity.
Other patient factors
The likelihood that patients will adhere to therapy or follow-up monitoring is important for drugs such as warfarin and insulin which have a low therapeutic index and where alternatives are less effective. Health beliefs and attitude to risk can influence the initial decision to prescribe or the choice of medicine. This is particularly obvious in long-term preventive therapy when benefits may be imperceptible. About half of patients adhere poorly to such treatments, emphasising the role of patient partnership in making rational prescribing decisions.
Prescriber factors influencing drug selection
Familiarity
Lack of familiarity of prescribers with medicines increases the chance of adverse outcomes, mandating continuing professional development. However, lack of experience should not impede the introduction of new, more rational prescribing practices.
Ease of follow-up
Some medicines require careful review and monitoring to ensure that safety is maximised or dose titration optimal. The ease with which these can be accomplished is important.
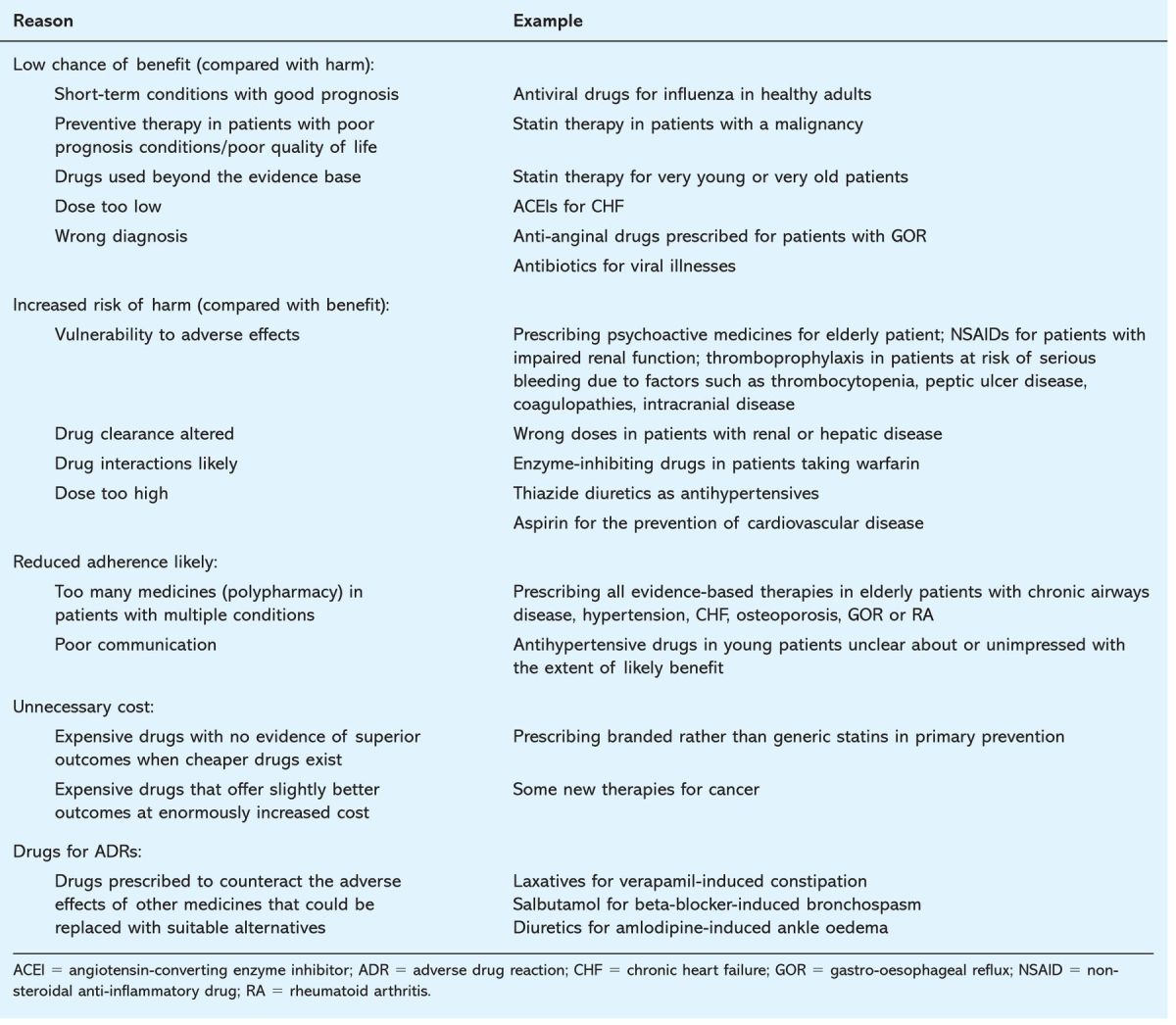
Examples of irrational prescribing
Rational prescribing aims to ensure that selection is not a simple formulaic linkage of drugs and doses to particular diagnoses, but involves individualising prescriptions as far as possible, taking account of the variables discussed above.
Table 3 offers some simple examples of irrational prescribing. They are illustrative only and do not acknowledge the complexity of real prescribing decisions. Prescribers commonly make probabilistic judgements that involve interpreting trial evidence in the light of specific circumstances such as patients’ wishes, availability of resources and previous adverse events. For instance, more expensive but equivalent medications may be justified if others have caused adverse effects or loss of confidence. Higher risk medicines may be acceptable if the potential benefit is estimated to be greater for an individual patient.
Table 3.
Examples of irrational prescribing.
Key points
Prescribing is a complex task that requires interpretation of evidence from clinical trials in light of individual patient factors
Rational prescribing describes a logical approach that includes making a diagnosis, estimating prognosis, establishing the goals of therapy, selecting the most appropriate treatment and monitoring the effects of the treatment
Patients should be involved in several of these stages and their beliefs, expectations and attitudes to risk will contribute to rational prescribing decisions
Pharmacogenetics will help to individualise prescribing choices but will not replace the need for an understanding of the clinical pharmacology underpinning the selection of commonly prescribed drugs
Personalised medicines: the future?
This article has discussed the traditional approach to prescribing in which individualised drug selection is based on evidence gathered from groups of similar patients mixed with best-guess judgements about the variability introduced by specific patient and drug factors. Recently, a new era of ‘personalised’ treatment has been predicted in which therapeutic choices will be individualised based on genetic variables affecting drug handling and action, allowing more specific prediction of outcomes. Indeed, pharmacogenetics is already being used to distinguish responders from non-responders (eg trastuzumab for HER2-overexpressing breast cancer) and to avoid adverse effects (eg HLA B∗5701 for abacavir hypersensitivity). (See accompanying article on pharmacogenetics.)
The impact of this approach may however be limited because many of the variables outlined in Table 2 are not affected by genetics. This suggests that rational prescribing will remain based on a firm grounding in the principles of clinical pharmacology.
Further reading
- 1.Aronson JK. Balanced prescribing. Br J Clin Pharmacol 2006;62:629–32. 10.1111/j.1365-2125.2006.02825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson JK. Changing beta-blockers in heart failure: when is a class not a class? Br J Gen Pract 2008;58:387–9. 10.3399/bjgp08X299317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audit Commission. A prescription for improvement. Toward more rational prescribing in general practice. London: Stationery Office, 1994. [Google Scholar]
- 4.Barber N. What constitutes good prescribing? BMJ 1995;310:923–5. 10.1136/bmj.310.6984.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Review. Arch Intern Med 1997;157:1531–6. [PubMed] [Google Scholar]
- 6.Blue JW, Colburn WA. Efficacy measures: surrogates or clinical outcomes? J Clin Pharmacol 1996;36:767–70. [DOI] [PubMed] [Google Scholar]
- 7.Bucher HC, Guyatt GH, Cook DJ, Holbrook A, McAlister FA. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. Evidence-Based Medicine Working Group. JAMA 1999;282:771–8. [DOI] [PubMed] [Google Scholar]
- 8.de Vries TP. Presenting clinical pharmacology and therapeutics: a problem based approach for choosing and prescribing drugs. Br J Clin Pharmacol 1993;35:581–6. 10.1111/j.1365-2125.1993.tb04185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries TP, Henning RH, Hogerzeil HV, Fresle DA. World Health Organization. Guide to good prescribing – a practical manual, 1994. WHO/DAP/94.11 www.who.int/medicinedocs/en/d/Jwhozip23e/
- 10.Hogerzeil HV. Promoting rational prescribing: an international perspective. Review. Br J Clin Pharmacol 1995;39:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GP Notebook – a UK medical reference on the world wide web. Rational prescribing. www.gpnotebook.co.uk/simplepage.cfm?ID=1362427972
- 12.Lesko LJ. Personalized medicine: elusive dream or imminent reality? Clin Pharmacol Ther 2007;81:807–16. 10.1038/sj.clpt.6100204 [DOI] [PubMed] [Google Scholar]
- 13.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353: 487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 14.Roland M, Torgerson D. Understanding controlled trials: what outcomes should be measured? BMJ 1998;317:1075. 10.1136/bmj.317.7165.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ 1996;312:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott DK, Ferner RE. ‘The strategy of desire’ and rational prescribing. Br J Clin Pharmacol 1994;37:217–9. 10.1111/j.1365-2125.1994.tb04265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodcock J. The prospects for ‘personalized medicine’ in drug development and drug therapy. Clin Pharmacol Ther 2007;81:164–9. 10.1038/sj.clpt.6100063 [DOI] [PubMed] [Google Scholar]