In order to grant a product licence for a medicine, the licensing authorities need to be satisfied that there is sufficient evidence to provide reassurance on pharmaceutical quality, therapeutic efficacy (how well it works in trials) and safety (lack of adverse effects). It is not their task to advise on effectiveness (how well it works in practice), value for money or the potential role (if any) that a new product might have in the NHS. Decisions on availability and use of new medicines are made at various levels in the NHS. At a national level, the National Institute for Health and Clinical Excellence (NICE), the All Wales Medicines Strategy Group and the Scottish Medicines Consortium provide advice, guidelines and directions. Many strategic health authorities have priority setting committees, primary care trusts may have area prescribing committees, hospitals have drug and therapeutics committees (DTCs), while individual departments and general practices may have their own formularies.
There is therefore the potential for duplication of effort and significant differences in the conclusions reached, leading to inequalities in the provision of care across the country (so-called ‘post-code’ prescribing). Evaluating technologies at a national level has the potential to reduce inequality and generally results in a high standard of assessment. However, not all drugs and technologies are currently covered, deliberations are slow to be published and local affordability remains problematic.
In 1997, the Labour Government set out its vision for the NHS, committing itself anew to the historic principle of the NHS that if you are ill or injured there will be a national health service there to help; and it will be based on need and need alone.
However, trusts in the UK have a statutory requirement under the 1977 NHS Act to ensure that their expenditure does not exceed their income. Clearly these two commitments could conflict and careful judgement is required to balance them. Finite healthcare budgets dictate that attention is paid to costs as well as needs. Hence, cost-effectiveness becomes important. In practice, the overriding consideration is affordability. An intervention that gains one extra year of life at an additional cost of £10,000 might be thought highly cost-effective, but might not be affordable if five million people merited treatment with it.
Clinical effectiveness versus cost-effectiveness
Decisions made by DTCs are based initially on considerations of clinical effectiveness and then on cost-effectiveness. The purpose of assessing the latter is to inform decision makers of the balance between costs and health gains in order to maximise health outcomes in the population. The aim is to minimise the opportunity cost. That is, the value of the output that would arise through the next best alternative use of resources available.
Estimates of cost-effectiveness are derived from economic evaluations: comparative analyses of two or more alternative courses of action (interventions) in terms of their costs and consequences:
Cost: the sum of the number of individual resource items used, each multiplied by its unit cost.
Consequences: the health outcomes (eg the impact of therapy on mortality, quality of life or both).
Methods of economic evaluation
All techniques of economic evaluation involve the same explicit consideration and calculation of the use of resources and overall costs, but each method handles consequences differently.
The perspective of an economic evaluation determines which costs are to be valued. The perspective might be narrow (eg a primary care organisation) or broad (eg the NHS or society). Not only is the cost of the intervention subject to economic evaluation but also the total costs related to treatment with that intervention including, for instance, hospitalisation, other interventions, blood tests and general practitioner visits.
Healthcare interventions often incur costs over a number of years, so timing is an important factor in many costings. Timing is accounted for in several ways:
All costs are valued in a base year, which normally reflects the latest available prices.
Capital costs are apportioned over the lifetime of the item (eg building, substantial equipment).
Future costs are discounted back to the base year – in general terms, reflecting a preference to put off costs rather than to pay immediately. NICE currently discounts both costs and benefits at 3.5% per annum.
Types of analysis
Cost-minimisation
Cost-minimisation analysis (CMA) requires robust evidence to show that two or more interventions have exactly the same health effects: that they are therapeutically equivalent in terms of health benefits and adverse effects. CMA can be used to compare branded and generic medicines or different formulations of the same drug but its practical applications are limited. However, in the absence of access to economic and health outcome data, many decisions made by DTCs are based (inappropriately) on CMAs.
Cost-effectiveness
Cost-effectiveness analysis (CEA) is appropriate when the size of the health effects of two or more interventions is not identical but are measured in the same units (eg life-years gained or symptom-free days).
Cost-utility
Cost-utility analysis (CUA) is the most useful form of economic evaluation and is appropriate when the health effects of two or more alternatives can be measured in terms of overall impact on quantity and quality of life. CUA is a special form of CEA in which the consequences are measured in terms of quality-adjusted life-years (QALYs).
Quality-adjusted life-years
QALYs are calculated by estimating the total life-years gained from a treatment, weighing each year (or part thereof) with a quality-of-life (‘utility’) score (0 for ‘dead’, 1 for ‘full health’). Various methods, including the EuroQol-5D questionnaire, can be used to quantify health-related quality of life to provide a single summary score.
The advantage of the QALY is that it incorporates quality and quantity of life in a common currency that allows comparison of interventions from different clinical areas. QALYs can therefore be compared for very different interventions, such as chemotherapy in advanced breast cancer, surgery for coronary artery bypass grafting and medicines for diabetes. For this reason, CUAs are the preferred form of economic evaluation in appraisals by NICE.
Modelling
Clinical trials do not usually capture all the data required for an economic analysis. Moreover, it is often appropriate to project the results of clinical trials beyond the time horizon of analysis to capture predicted lifetime costs and benefits. Economic analyses are essentially mathematical models used to compile data from various sources and to test the robustness of underlying assumptions and uncertainties.
The most common forms of economic models are decision analyses, represented schematically as decision trees, and Markov models, which are helpful for modelling the progression of chronic diseases. Within a Markov model, a disease is divided into health states (eg remission, progression, death). Each individual is given a probability of moving from one state to another during a chosen period of time. Estimates of the use of resources and health effects are also attached to each state. The model is then run to produce long-term estimates of cost-effectiveness in hypothetical defined patient cohorts.
Criteria for decision making
Judgement about whether any medicine represents good value for money (and whether it will therefore be recommended for use by a decision-making body) depends on whether the incremental cost-effectiveness ratio (ICER) is considered acceptable. ICERs are calculated by ranking treatments in descending order of effectiveness, then dividing the difference in costs between each intervention by the difference in their effectiveness (QALYs). In practice, NICE considers that healthcare interventions costing less than £20,000–30,000 per QALY gained are cost-effective. There are also special dispensations for appraising end-of-life treatments for rare conditions, accepting that it may be considered appropriate to spend more per QALY in such cases.
Conclusions
The above discussion seems to suggest that costs, benefits and harms for healthcare interventions can be neatly ascribed and hence rational prescribing decisions made. In reality, there are many potential confounders:
clinical trials vary in their outcomes and often overestimate benefits and significantly underestimate harms
trial participants may not reflect the age, ethnicity, sex or comorbidities of patients in general,
capturing all the costs, both to the NHS and society, is fraught with problems.
Key points
Decisions by drug and therapeutics committees on the availability of medicines should be informed by evidence on cost-effectiveness as well as clinical effectiveness and safety
Economic evaluations are quantitative methods by which health economists assess the cost-effectiveness of medicines and other healthcare interventions
The usual measure of health outcome in economic evaluations is the quality-adjusted life-year (QALY) which allows comparisons to be made across the full range of clinical areas
A medicine is generally considered to be cost-effective if each additional QALY gained by using it costs less than £20,000–30,000
There are sometimes reasons for approving the use of a medicine (or its inclusion in a formulary) despite economic evidence that it is not cost-effective
Nevertheless, the discipline of health economic analysis has contributed significantly to healthcare over the last decade and provides a starting point for rational decision making by DTCs. The cost per QALY should never be taken in isolation. Rather, a committee should note it and then ask ‘is there any reason to suppose that we should make an exception in this case?’ Table 1 presents examples of cases in which equity may be considered to be more important than efficiency.
Table 1.
Examples of cases in which decisions have emphasised factors unrelated to cost-effectiveness.
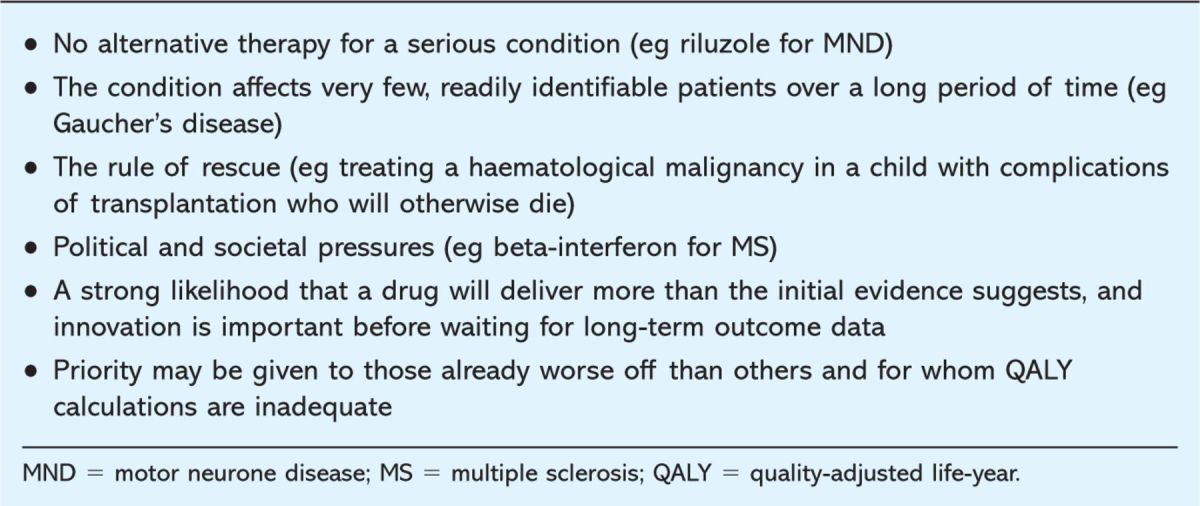
These are matters of judgement. It is not surprising that at times those who care for patients or the patients themselves may disagree with the recommendation of a body such as NICE. It is therefore important that the underlying principles of how decisions are made are explicit and open to challenge. All DTCs should have a mechanism for dealing with exceptions to policy statements and re-evaluate decisions when circumstances and evidence change.
Further reading
- 1.All Wales Medicines Strategy Group. www.wales.nhs.uk/awmsg
- 2.Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of heath care: selecting the appropriate approach. J Health Serv Res Policy 2004;9:110–8. [DOI] [PubMed] [Google Scholar]
- 3.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275–83. 10.1136/bmj.313.7052.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes, 3rd edn. Oxford: Oxford University Press, 2005. [Google Scholar]
- 5.Elliott R, Payne K. Essentials of economic evaluation in healthcare. London: Pharmaceutical Press, 2005. [Google Scholar]
- 6.National Institute for Health and Clinical Excellence. www.nice.org.uk
- 7.National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal, 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf [PubMed]
- 8.Scottish Medicines Consortium. www.scottishmedicines.org.uk/smc/
- 9.Sculpher M, Fenwick E, Claxton K. Assessing quality in decision analytic cost-effectiveness models. A suggested framework and example of application. Pharmacoeconomics 2000;17:461–77. [DOI] [PubMed] [Google Scholar]
- 10.Walley T, Haycox A, Boland A. (eds). Pharmacoeconomics, 1st edn. Edinburgh: Churchill Livingstone, 2004. [Google Scholar]


