Abstract
Therapeutic glucocorticoids are widely used to treat a variety of inflammatory conditions. However, the beneficial anti-inflammatory effects of glucocorticoids are limited by their detrimental effects on bone, including decreased bone density and increased fracture risk. Glucocorticoids adversely affect bone because they inhibit the amount of bone formed by osteoblasts. Surprisingly, through the expression of the 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) enzyme, osteoblasts can generate active glucocorticoids (cortisol and/or prednisolone) from their inactive counterparts (cortisone and/or prednisone). 11β-HSD1 activity in an individual predicts the impact of glucocorticoids on bone. 11β-HSD1 expression within bone also increases with age and inflammation. This implicates locally produced glucocorticoids in age-related and inflammation-associated osteoporosis. Glucocorticoids are also generated by synovial tissue through the expression of 11β-HSD1. Activity increases with joint inflammation and could represent a local anti-inflammatory system. The recognition that peripheral tissues generate glucocorticoids suggests that, for conditions associated with ageing or inflammation, one should consider glucocorticoid activity beyond the circulation.
Key Words: osteoporosis, glucocorticoids, 11β-hydroxysteroid dehydrogenase, inflammation, arthritis
Introduction
When an endocrinologist thinks about glucocorticoid excess, they will generally think of Cushing's disease. This condition of endogenous glucocorticoid excess exemplifies all of the adverse features of glucocorticoids, including central obesity, myopathy, striae, bone fracture and immunocompromise. However, this condition is rare and, nowadays, glucocorticoid excess results most commonly from the use of therapeutic steroids. Introduced during the 1950s as broad-spectrum anti-inflammatory drugs, the use of these medications has grown almost exponentially and they are now used in most branches of medicine. As a testament to how ‘good’ these drugs are, data from the UK show that approximately 1% of the population are taking oral glucocorticoids at any time and this figure increases to almost 3% in the elderly.1 Many more people are taking these drugs intermittently or via topical routes. One of the main limitations of glucocorticoid therapy is the adverse effect that they have on the skeleton. They can cause bone loss (i.e. osteoporosis) and fractures: features collectively referred to as glucocorticoid-induced osteoporosis (GIOP).
Glucocorticoid-induced osteoporosis
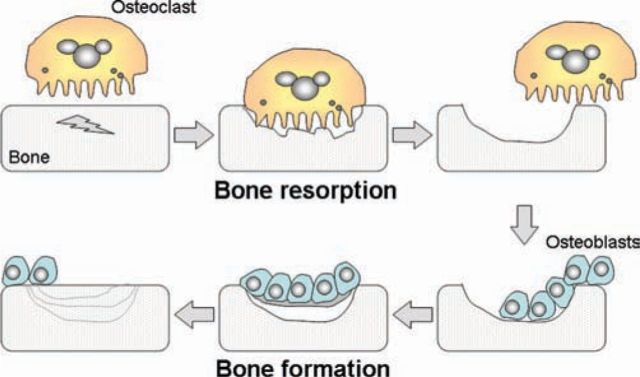
Epidemiological studies have established that fracture risk with oral glucocorticoids increases in a dose-dependent manner.2 These studies also highlight that the risk of fracture increases quickly after starting glucocorticoids and also decreases rapidly after stopping. Although glucocorticoids have a variety of direct and indirect effects on bone, the most important effect appears to be through interference with the way in which the body repairs and renews the skeleton.3 In contrast to its post-mortem appearance, the living skeleton is a dynamic organ undergoing constant breakdown and reformation at the microscopic level. The main cells within bone are the osteoblasts and osteoclasts. Osteoblasts are bone-forming cells, whereas osteoclasts are bone-resorbing cells. Both cell types can have independent roles; for example, osteoblasts are important in allowing bones to increase in size, whereas osteoclasts have an immune and/or phagocytic role in the removal of dead or diseased bone. However, in the adult skeleton, these cells primarily work together in a highly coordinated process referred to as bone remodelling (Fig 1). At the beginning of a remodelling cycle, osteoclasts excavate a packet of bone before they move away or die. They are then replaced by osteoblasts that lay down osteoid (unmineralised bone) and regulate its subsequent mineralisation. At the end of a remodelling cycle, there is no change in the net amount of bone tissue. Glucocorticoids interfere with the bone remodelling cycle by profoundly reducing the ability of osteoblasts to replace bone excavated by osteoclasts.3 The switching off of bone formation, with continued bone resorption by osteoclasts, leads to a substantial decrease in bone density and an impairment in the microscopic architecture of bone.
Fig 1.
The bone remodelling cycle. Microscopic damage within bone is repaired by the coordinated action of osteoclasts and osteoblasts. Osteoclasts initially remove a packet of bone. They are then replaced by osteoblasts, which lay down matrix (osteoid) that subsequently mineralises.
Current treatments to protect against GIOP primarily target the relative excess of bone resorption by using bisphosphonates. These drugs are highly effective at ‘freezing’ the bone by reducing remodelling and have demonstrated effectiveness in randomised controlled trials.4 However, there are concerns that prolonged use of these drugs, particularly in states of poor bone formation, could prevent the normal repair of microdamage to bone that occurs during daily activities. The recent recognition of ‘atypical subtrochanteric fractures’ as a possible consequence of long-term bisphosphonate and glucocorticoid use suggests that this concern is real.5 An alternative treatment for GIOP, teriparatide, directly counters the main problem in GIOP by stimulating bone formation. This treatment is superior to bisphosphonates in protecting against vertebral fracture in this setting.6 However, the drug is more expensive than bisphosphonates and needs to be given as a daily subcutaneous injection.
Endogenous glucocorticoids in bone physiology
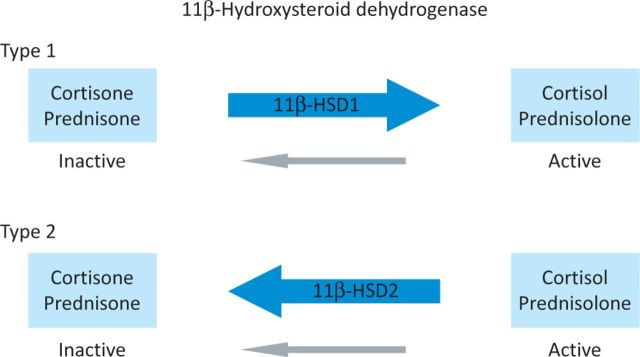
It is unclear why bone should be so sensitive to glucocorticoids. Additionally, clinical experience indicates that this sensitivity varies considerably among individuals, but the basis for this variability is unknown. Insights into this area have come from an improved understanding of glucocorticoid physiology and, in particular, the local metabolism of glucocorticoids. At a tissue level, glucocorticoids are interconverted between inactive cortisone and active cortisol.7 Similar metabolism occurs for the synthetic glucocorticoids, such as prednisone (inactive) and prednisolone (active). The enzymes that catalyse these reactions are the 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes (Fig 2). The 11β-HSD1 enzyme is intrinsically bidirectional but in vivo primarily acts to activate glucocorticoids. 11β-HSD1 is present in high amounts in fat and liver tissues, where it amplifies the level of active glucocorticoid. By contrast, the 11β-HSD2 enzyme is a powerful inactivator of glucocorticoids and is found primarily in the kidney, where it protects the mineralocorticoid receptor from occupation by cortisol. The importance of 11β-HSD1 is emphasised by rare patients who lack activity of this enzyme. These individuals are unresponsive to cortisone or prednisone treatment (because they cannot activate these steroids in the body), but remain sensitive to cortisol and prednisolone therapy.7 The activity of 11β-HSD1 also modifies the clinical presentation of Cushing's disease.8 We hypothesised that differences in 11β-HSD enzyme expression would explain the differences in sensitivity of bone to glucocorticoids. Therefore, we characterised the expression of 11β-HSD enzymes in human bone.9
Fig 2.
The 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes. These enzymes interconvert hormonally inactive glucocorticoids, such as cortisone and prednisone, with their active counterparts, cortisol and prednisolone, respectively.
Initially, we incubated small pieces of bone, freshly isolated from discarded orthopaedic specimens, with medium containing various concentrations of either cortisone or cortisol. The medium was then removed and the conversion of steroids measured. We found consistently that bone was able to interconvert glucocorticoids and that this conversion was greatest under conditions that favoured the 11β-HSD1 enzyme. Supporting this observation, immunohistochemistry and in situ hybridisation demonstrated expression of 11β-HSD1 but not the 11β-HSD2 enzyme. The expression of 11β-HSD1 was primarily localised to osteoblasts. The functional consequences of 11β-HSD1 expression were tested in osteoblastic cells with stable transfection of the enzyme.10 Cells that did not express 11β-HSD1 were unresponsive to cortisone. However, the transfection of 11β-HSD1 resulted in these cells being sensitive to this glucocorticoid and, when expression levels were similar to those seen in vivo in some of the situations discussed below, cortisone had an equivalent effect on the cell to that of cortisol.
Local glucocorticoid metabolism in GIOP
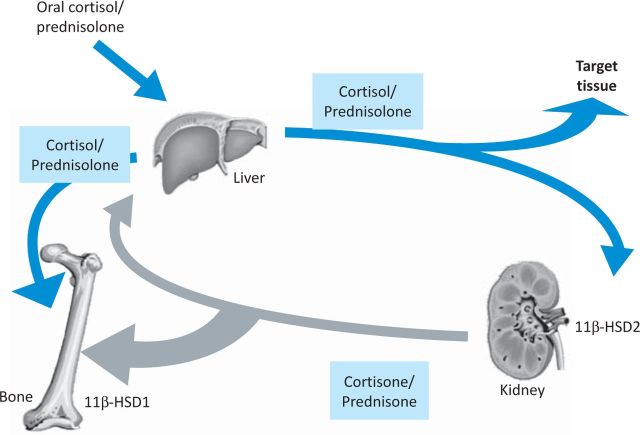
These observations led us to propose a model in which endogenous and therapeutic glucocorticoids affect bone (Fig 3). There is a direct impact of circulating glucocorticoids upon bone. However, in addition, there is a second impact from local activation of inactive glucocorticoids that are present in high concentrations within the circulation. To examine the relative importance of these two pathways in GIOP, we conducted a clinical trial using healthy volunteers.11 At baseline, a measurement of total body 11β-HSD1 activity and cortisol secretion was made based on a 24 h urine collection. Levels of biochemical markers of bone formation and resorption were also made. Subjects were then given prednisolone 10 mg per day for one week with repeat bone markers taken at day seven. As expected, there was a substantial fall in markers of bone formation (osteocalcin and procollagen I N-terminal propeptide (PINP)) in all subjects. However, the extent of fall varied considerably among individuals and was unrelated to the amount of cortisol produced at baseline or to the level of prednisolone within the circulation during treatment. However, it was strongly correlated with the baseline level of 11β-HSD1 activity. For osteocalcin, a fall of approximately 60% was seen in individuals with high 11β-HSD1 activity, compared with approximately 20% in individuals with low 11β-HSD1 activity. This indicated that an individual's level of 11β-HSD1 activity was the major factor in determining the effect of glucocorticoids on bone formation.
Fig 3.
Pathways by which glucocorticoids impact on bone. In addition to a direct effect of circulating active glucocorticoids on bone, there is an additional effect of glucocorticoids regenerated from inactive precursors. The magnitude of the indirect effect depends on the level of expression of the 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) enzyme.
Local glucocorticoid metabolism in age-related osteoporosis
The ability of bone to increase local glucocorticoid levels suggests that this system has implications outside of the setting of GIOP. Skeletal ageing shares many features with GIOP. Ageing is characterised by not only an increase in bone resorption, but also a progressive deficit in bone formation relative to bone resorption. This imbalance in the remodelling cycle leads to progressive bone loss. Although these features are reminiscent of GIOP, the circulating level of glucocorticoids does not change greatly with age. We explored the hypothesis that age-related reduction in bone formation might be the result of increased local glucocorticoid metabolism. We measured the 11β-HSD1 activity in osteoblasts grown from chips of bone tissue derived from individuals across a wide range of ages.12 We found a strong correlation between the ability of these osteoblasts to activate glucocorticoids and the age of the bone donor. Osteoblasts from older individuals generated approximately four times the amount of cortisol relative to those from young individuals. A similar age-related increase in 11β-HSD1 expression in osteoblasts has more recently been reported in vivo in mice.13 These data indicate that many of the features of skeletal ageing are in fact the result of a highly localised, endogenous form of GIOP.
Local glucocorticoid metabolism in inflammation-related osteoporosis
Another condition characterised by an impairment of bone formation relative to resorption is the periarticular loss of bone seen in inflammatory arthritis.14 It is known that high levels of proinflammatory cytokines produced during inflammation stimulates bone resorption. However, inflammatory cytokines also suppress the amount of bone formed and this uncoupling of the remodelling cycle leads to bone loss if inflammation persists. We hypothesised that this uncoupling could be because of locally produced glucocorticoids, and examined the ability of pro-inflammatory cytokines to upregulate production of active glucocorticoids in osteoblasts.15 We found that both tumour necrosis factor (TNF)-α and interleukin (IL)-1β potently stimulated 11β-HSD1 expression and activity in primary osteoblasts. This suggests that locally produced glucocorticoids have a role in inflammation-associated osteoporosis.
Why does bone make glucocorticoids?
Accumulating experimental data indicate that locally produced glucocorticoids have an important role in several situations where bone formation is uncoupled from bone resorption. The finding that osteoblasts are able to generate active glucocorticoids seems surprising, given the known detrimental effects of glucocorticoids on bone. However, it must be appreciated that, in some situations, it is not appropriate for bone formation and resorption to be coupled. An example would be inflammation or infection in bone. In this situation, the role of the osteoclasts would ideally switch from bone remodelling to a primarily immune and/or phagocytic role. In this situation, the formation of new bone is inappropriate until the underlying problem within the bone is resolved. Increased 11β-HSD1 appears to be a physiological uncoupler of bone formation in this situation. This hypothesis is supported by the bone phenotype of the 11β-HSD1-knockout mouse, in which osteoblasts cannot make active glucocorticoids.17 Under normal conditions, the bone phenotype appears normal. However, in response to experimental arthritis, aberrant new bone is formed adjacent to the areas of greatest inflammation. However, this beneficial action of 11β-HSD1 in maintaining overall bone architecture is likely to lead to bone loss if inflammation is prolonged.
Endogenous glucocorticoids in inflammatory arthritis
During our studies to examine glucocorticoid production in bone, we attempted to measure glucocorticoid levels in synovial fluid from patients with inflammatory arthritis. To our surprise, we found that the level of cortisol was higher and the level of cortisone was much lower than we expected.18 This indicated substantial 11β-HSD activity that was unlikely to be entirely from bone. Further experiments using synovial tissue and cells isolated from synovial tissue indicated that synovial tissue itself has considerable expression of 11β-HSD1 and is able to generate active glucocorticoids.17,18 Synovial tissue contains a variety of cell types, including synovial fibroblasts, macrophages and lymphocytes. Using immunohistochemistry, the cell type with the most consistent positive expression of 11β-HSD1 was the synovial fibroblast, although some expression was also seen in macrophages. As in bone tissue, there was a close correlation between 11β-HSD1 and inflammation. Glucocorticoid generation in synovial tissue explants obtained from patients with established rheumatoid arthritis was strongly correlated with the patients’ erythrocyte sedimentation rate (ESR) measured immediately preoperatively.17 Systemic measures of 11β-HSD1 based on urinary corticosteroid metabolite excretion were also raised in patients with rheumatoid arthritis relative to patients with non-inflammatory joint conditions. In ongoing work, we have found that anti-TNF treatment reverses increased 11β-HSD1 activity back towards normal levels.
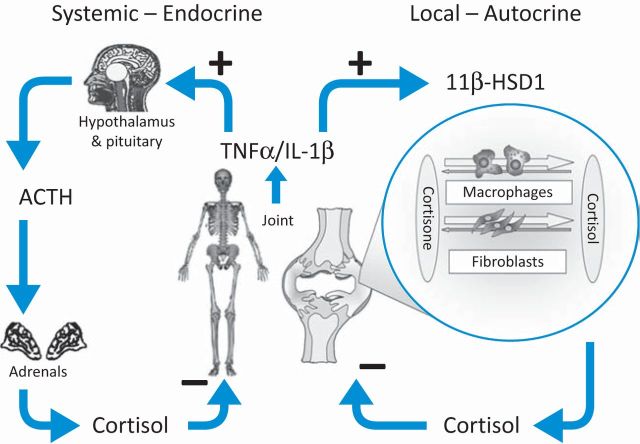
These findings indicate that there are two endogenous glucocorticoid-based systems by which joint inflammation can be regulated (Fig 4). First, there is the hypothalamic–pituitary–adrenal axis, in which systemic inflammation stimulates the production of adrenocorticotropic hormone (ACTH) from the pituitary, which in turn stimulates adrenal cortisol production.19,20 This circulating cortisol is able to dampen joint inflammation. Additionally, there is a second system at a local level, whereby local inflammation stimulates an increase in synovial tissue glucocorticoid production. This locally produced cortisol can act in an autocrine and/or paracrine manner to reduce local inflammation. It is likely that, for many transient inflammations of joint tissue, these mechanisms are sufficient to control inflammation. However, it appears that these mechanisms are not always entirely effective and inflammatory arthritis can overwhelm the ability of the body to produce glucocorticoids. We are currently exploring how these two anti-inflammatory systems interact and whether abnormalities of these responses underlie the development, severity or persistence of inflammatory arthritis.
Fig 4.
Systemic and local regulation of joint inflammation. Pro-inflammatory cytokines released during joint inflammation lead to an increase in the level of cortisol within the systemic circulation through activation of the hypothalamic–pituitary–adrenal axis (HPA) axis. These cytokines also cause an activation of local glucocorticoid generation through an increase in 11β-hydroxysteroid dehydrogenase 1 (11β–HSD1) activity. ACTH = adrenocorticotropic hormone; IL-1β = interleukin 1β; TNFα = tumour necrosis factor α.
Conclusion
Our research suggests that bone is so sensitive to therapeutic glucocorticoids because endogenous glucocorticoids are used to uncouple bone formation from resorption. Endogenous glucocorticoid production within bone is also involved in age-related and inflammation-associated osteoporosis. This raises the possibility that drugs that inhibit 11β-HSD1 activity would have the potential to build up bones by altering the bone remodelling cycle in favour of excess bone formation. Inflamed synovial tissue also generates active glucocorticoids. This probably modifies the expression of inflammatory arthritis and could be a factor in determining whether an inflammatory response persists or resolves. The recognition that peripheral tissues can generate glucocorticoids suggests that, for other medical conditions associated with ageing or inflammation, one should consider glucocorticoid activity beyond the circulation.
Acknowledgements
The work on which this paper was based was funded by the Medical Research Council, Arthritis Research UK and an unrestricted GlaxoSmithKline Clinician Scientist Fellowship. I would like to acknowledge and thank all of the individuals that have contributed to the studies on which this paper is based, in particular, Karim Raza, Chris Buckley and Paul Stewart.
References
- 1.van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids in the United Kingdom. QJM 2000;93:105–11 10.1093/qjmed/93.2.105 [DOI] [PubMed] [Google Scholar]
- 2.van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993–1000 [DOI] [PubMed] [Google Scholar]
- 3.Cooper MS. Sensitivity of bone to glucocorticoids. Clin Sci 2004;107:111–23 10.1042/CS20040070 [DOI] [PubMed] [Google Scholar]
- 4.Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 2010;6:82–8 10.1038/nrrheum.2009.259 [DOI] [PubMed] [Google Scholar]
- 5.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 2008;358:1304–6 10.1056/NEJMc0707493 [DOI] [PubMed] [Google Scholar]
- 6.Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 2007;357:2028–39 [DOI] [PubMed] [Google Scholar]
- 7.Cooper MS, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J Clin Endocrinol Metab 2009;94:4645–54 [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson JW, Draper N, Mackie J, et al. Absence of cushingoid phenotype in a patient with Cushing's disease due to defective cortisone to cortisol conversion. J Clin Endocrinol Metab 2002;87:57–62 10.1210/jc.87.1.57 [DOI] [PubMed] [Google Scholar]
- 9.Cooper MS, Walker EA, Bland R, et al. Expression and functional consequences of 11b-hydroxysteroid dehydrogenase activity in human bone. Bone 2000;27:375–81 [DOI] [PubMed] [Google Scholar]
- 10.Rabbitt E, Lavery GG, Walker EA, et al. Pre-receptor regulation of glucocorticoid action by 11b-hydroxysteroid dehydrogenase: a novel determinant of cell proliferation. FASEB J 2002;16:36–44 [DOI] [PubMed] [Google Scholar]
- 11.Cooper MS, Blumsohn A, Goddard PE, et al. 11b-hydroxysteroid dehydrogenase type 1 activity predicts the effects of glucocorticoids on bone. J Clin Endocrinol Metab 2003;88:3874–7 [DOI] [PubMed] [Google Scholar]
- 12.Cooper MS, Rabbitt EH, Goddard PE, et al. Osteoblastic 11b-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res 2002;17:979–86 [DOI] [PubMed] [Google Scholar]
- 13.Weinstein RS, Wan C, Liu Q, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 2010;9:147–61 10.1111/j.1474-9726.2009.00545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy R, Cooper MS. Bone loss in inflammatory disorders. J Endocrinol 2009;201:309–20 10.1677/JOE-08-0568 [DOI] [PubMed] [Google Scholar]
- 15.Cooper MS, Bujalska I, Rabbitt E, et al. Modulation of 11b-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 2001;16:1037–44 [DOI] [PubMed] [Google Scholar]
- 16.Coutinho AE, Gray M, Brownstein DG, et al. 11beta-hydroxysteroid dehydrogenase type 1, but not type 2, deficiency worsens acute inflammation and experimental arthritis in mice. Endocrinology 2012;153:234–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy R, Rabbitt EH, Filer A, et al. Local and systemic glucocorticoid metabolism in inflammatory arthritis. Ann Rheum Dis 2008;67:1204–10 10.1136/ard.2008.090662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy RS, Filer A, Cooper MS, et al. Differential expression, function and response to inflammatory stimuli of 11b-hydroxysteroid dehydrogenase type 1 in human fibroblasts: a mechanism for tissue-specific regulation of inflammation. Arthritis Res Ther 2006;8:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003;348:727–34 10.1056/NEJMra020529 [DOI] [PubMed] [Google Scholar]
- 20.Raza K, Hardy R, Cooper MS. The 11beta-hydroxysteroid dehydrogenase enzymes–arbiters of the effects of glucocorticoids in synovium and bone. Rheumatology 2010;49:2016–23 [DOI] [PubMed] [Google Scholar]