Lesson
In September 2010, a 55-year-old lady presented to her GP following a fortnight's holiday in Mana Pools National Park, Zimbabwe. She had taken antimalarial prophylaxis. On returning to the UK she developed fevers, malaise and was non-specifically ‘unwell’. According to her flat-mate, ‘she could feed the cat and make cheese-on-toast, but was good for little else’. As the patient had previously experienced a depressive psychosis, her GP suspected that her symptoms were psychiatric and commenced treatment with antipsychotic and antidepressant medications.
The patient continued to deteriorate and two weeks after her initial presentation a blood film was performed to exclude malaria. It revealed trypanosomes. The patient was diagnosed with human African trypanosomiasis (HAT), also known as sleeping sickness, and was transferred to the Hospital for Tropical Diseases.
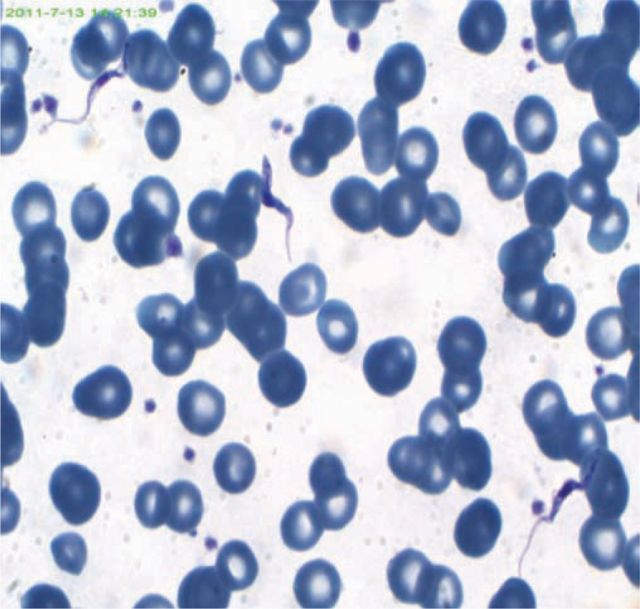
On arrival, the patient was able to converse but was uninhibited, had a fluctuating orientation and had difficulty with tasks such as operating her mobile phone. She recalled receiving multiple tsetse fly bites while kayaking in Zimbabwe. She had a grape-sized submandibular lymph node, but no chancre. Daytime somnolence and urinary incontinence were observed. Initial investigations revealed haemoglobin 7.5 g/l, white cell count 2.75 × 109/l, C-reactive protein 122 mg/l, albumin 19 g/l and normal renal and liver function. Viable motile protozoa were seen on a wet-preparation. Thick and thin films with modified rapid Field's staining revealed numerous trypanosomes (Fig 1). The patient was presumed, on the basis of the country of acquisition and her acute presentation, to have been infected by Trypanosoma brucei rhodesiense. Her T.b.rhodesiense immunofluorescent antibody test (IFAT) was positive at one in 200.
Fig 1.
Modified Rapid Field's-stained thin film blood smear from the patient, revealing Trypanosoma brucei. Magnification 1000x.
Suramin was commenced to treat stage I haemolymphatic T.b.rhodesiense. Cerebrospinal fluid (CSF) examination on the 13th day of treatment (when the peripheral parasitaemia had resolved) demonstrated 14 lymphocytes/μl, with no trypanosomes seen. World Health Organisation guidelines advocate treatment for stage II encephalopathic T.b.rhodesiense if the CSF white cell count is greater than 5/μl, and therefore melarsoprol with adjunctive prednisolone was commenced (suramin cannot penetrate the blood brain barrier).1 Subsequent IFAT CSF analysis did not detect trypanosome antibodies and it is questionable whether there was central nervous system (CNS) involvement. Following full resolution of symptoms, the patient was discharged from hospital in November 2010. Unfortunately, she developed a bilateral pedal paraesthesia, which was most likely suramin-induced, and an axonal peripheral neuropathy was confirmed by nerve conduction studies.
Discussion
Human African trypanosomiasis is a protozoan parasitic disease caused by Trypanosoma brucei which is spread by the tsetse fly, thus limiting its transmission to sub-Saharan Africa. It is a disease of rural, poor and disenfranchised communities in Africa, with an estimated 50,000 to 70,000 cases occurring annually. It only sporadically affects short-term tourists who visit affected areas.2 T.b.rhodesiense occurs in east and southern Africa. T.b.gambiense is endemic to west and central Africa.
HAT has two disease stages: an initial haemolymphatic stage and a subsequent meningoencephalitic stage, during which the parasites invade the CNS. In cases of T.b.rhodesiense infection, CNS involvement occurs acutely, after weeks or months. T.b.gambiense causes a more insidious course, with CNS involvement occurring months to years after the initial inoculation. CNS infection is characterised by deregulation of the circadian sleep–wake cycle, movement disorders, seizures and, without treatment, the invariable progression to coma and death. Psychiatric and behavioural disorders, including apathy and inactivity, aggression and psychosis, can also be observed in people with meningoencephalitic HAT.3 In rural Africa, such presentations are frequently interpreted by the local community as the afflicted being possessed by evil spirits, whereas in the west, people who have this disease have been mistakenly admitted to psychiatric institutions.4 Both strains of HAT are difficult to diagnose and currently available treatments are less than ideal.
In the case described, the patient was suspected of having stage II encephalopathic T.b.rhodesiense, for which the recommended treatment is intravenous melarsoprol. Melarsoprol is an arsenic-containing drug that should not be commenced without confirmation of cerebral infection as it causes an irreversible encephalopathy in 10% of those who receive it. This encephalopathy is fatal in 50% of those who incur it. Administration of prednisolone can reduce the risk of encephalopathy5, but the development of less toxic drugs for this neglected tropical disease is clearly long overdue. Standard practice is to administer suramin until the peripheral parasitaemia has cleared, before considering a lumbar puncture. This prevents both the mechanical dissemination of parasites from blood to CSF and the false diagnosis of stage II disease if the CSF is inadvertently contaminated with blood. In our patient, using this precaution resulted in a 13-day delay before the CSF was examined. In the field in Africa, a lumbar puncture is usually performed after the first dose of suramin. The investigation and management of HAT remains controversial, largely because of the toxicity of melarsoprol.
Conclusion
‘Psychiatric’ symptoms in a patient returning from sub-Saharan Africa indicate a protozoan infection of the CNS until proven otherwise. A competent examination of a blood film is required in the first instance. The causative organism in this case was the trypanosome, which only rarely infects travellers, rather than the more commonly encountered malarial parasite.
References
- 1.World Health Organization Report of a World Health Organisation expert committee. Control and surveillance of African trypanosomiasis. 1998;881Geneva: 114.Technical Report Series 1998 [PubMed] [Google Scholar]
- 2.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet 2010;375:148–59 [DOI] [PubMed] [Google Scholar]
- 3.Kennedy PG. Human African trypanosomiasis – neurological aspects. J Neurol 2006;253:411–6 10.1007/s00415-006-0093-3 [DOI] [PubMed] [Google Scholar]
- 4.Bedat-Millet AL, Charpentier S, Monge SMF, Woimant F. Psychiatric presentation of human African trypanosomiasis: overview of diagnostic pitfalls, interest of difluoromethylornithine treatment and contribution of magnetic resonance imaging. Rev Neurol 2000;156:505–9 [PubMed] [Google Scholar]
- 5.Pepin J, Milford F, Mpia B, et al. An open clinical trial of nifurtimox for arsenic-resistant Trypanosoma brucei gambiense sleeping sickness in central Zaire. Trans R Soc Trop Med Hyg 1989;83:514–7 [DOI] [PubMed] [Google Scholar]