Abstract
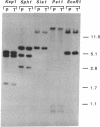
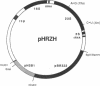
A vector-transformation system is described that permits replacement of a portion of the single rRNA operon of the archaeon Halobacterium halobium with a homologous fragment from a vector-borne gene. The vector construct contains three functional sections: (i) an entire H. halobium rRNA operon with two selective mutations in the 23S rRNA gene, the substitutions of A----G at position 1159 conferring resistance to thiostrepton and C----U at position 2471 conferring resistance to anisomycin; (ii) the complete pHSB1 plasmid from Halobacterium sp. SB3, which interferes with vector maintenance in the transformed halobacterial cells; and (iii) a segment of the pBR322 plasmid that permits vector replication in Escherichia coli. Transformation of H. halobium with the vector plasmid generates cells resistant to both anisomycin and thiostrepton that can be selected for, and discriminated from spontaneous mutants, by a two-step selection procedure. After transformation, the plasmid recombines homologously with the chromosome so that the plasmid-borne rDNA segment with resistance markers substitutes for the corresponding region of the chromosomal rRNA operon, and the transforming plasmid is lost. Eventually, this leads to a homogeneous population of the mutant ribosomes in the cell. Other mutations that are engineered in the vector-borne rRNA sequences can be transferred to the chromosomal rRNA operon concomitantly with the selective markers. The system has considerable potential for ribosomal engineering.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaseio U., Pfeifer F. Transformation of Halobacterium halobium: development of vectors and investigation of gas vesicle synthesis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6772–6776. doi: 10.1073/pnas.87.17.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Daniels C. J., Reeve J. N. Gene structure, organization, and expression in archaebacteria. Crit Rev Microbiol. 1989;16(4):287–338. doi: 10.3109/10408418909105479. [DOI] [PubMed] [Google Scholar]
- Chant J., Dennis P. Archaebacteria: transcription and processing of ribosomal RNA sequences in Halobacterium cutirubrum. EMBO J. 1986 May;5(5):1091–1097. doi: 10.1002/j.1460-2075.1986.tb04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. W., Lam W. L., Charlebois R. L., Schalkwyk L. C., Doolittle W. F. Transformation methods for halophilic archaebacteria. Can J Microbiol. 1989 Jan;35(1):148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- Cline S. W., Schalkwyk L. C., Doolittle W. F. Transformation of the archaebacterium Halobacterium volcanii with genomic DNA. J Bacteriol. 1989 Sep;171(9):4987–4991. doi: 10.1128/jb.171.9.4987-4991.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover R. K., Doolittle W. F. Characterization of a gene involved in histidine biosynthesis in Halobacterium (Haloferax) volcanii: isolation and rapid mapping by transformation of an auxotroph with cosmid DNA. J Bacteriol. 1990 Jun;172(6):3244–3249. doi: 10.1128/jb.172.6.3244-3249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. The functional role of ribosomal RNA in protein synthesis. Cell. 1989 May 19;57(4):525–529. doi: 10.1016/0092-8674(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Leffers H., Christensen A., Andersen H., Garrett R. A. Structure and accessibility of domain I of Escherichia coli 23 S RNA in free RNA, in the L24-RNA complex and in 50 S subunits. Implications for ribosomal assembly. J Mol Biol. 1987 Jul 5;196(1):125–136. doi: 10.1016/0022-2836(87)90515-8. [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Dalgaard J., Larsen N., Kjems J., Mankin A. S. Archaeal rRNA operons. Trends Biochem Sci. 1991 Jan;16(1):22–26. doi: 10.1016/0968-0004(91)90011-j. [DOI] [PubMed] [Google Scholar]
- Hackett N. R., DasSarma S. Characterization of the small endogenous plasmid of Halobacterium strain SB3 and its use in transformation of H. halobium. Can J Microbiol. 1989 Jan;35(1):86–91. doi: 10.1139/m89-013. [DOI] [PubMed] [Google Scholar]
- Hofman J. D., Lau R. H., Doolittle W. F. The number, physical organization and transcription of ribosomal RNA cistrons in an archaebacterium: Halobacterium halobium. Nucleic Acids Res. 1979 Nov 10;7(5):1321–1333. doi: 10.1093/nar/7.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. L., Dyall-Smith M. L. A plasmid vector with a selectable marker for halophilic archaebacteria. J Bacteriol. 1990 Feb;172(2):756–761. doi: 10.1128/jb.172.2.756-761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui I., Dennis P. P. Characterization of the ribosomal RNA gene clusters in Halobacterium cutirubrum. J Biol Chem. 1985 Jan 25;260(2):899–906. [PubMed] [Google Scholar]
- Hummel H., Böck A. 23S ribosomal RNA mutations in halobacteria conferring resistance to the anti-80S ribosome targeted antibiotic anisomycin. Nucleic Acids Res. 1987 Mar 25;15(6):2431–2443. doi: 10.1093/nar/15.6.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel H., Böck A. Thiostrepton resistance mutations in the gene for 23S ribosomal RNA of halobacteria. Biochimie. 1987 Aug;69(8):857–861. doi: 10.1016/0300-9084(87)90212-4. [DOI] [PubMed] [Google Scholar]
- Kagramanova V. K., Derckacheva N. I., Mankin A. S. The complete nucleotide sequence of the arcaebacterial plasmid pHSB from Halobacterium, strain SB3. Nucleic Acids Res. 1988 May 11;16(9):4158–4158. doi: 10.1093/nar/16.9.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagramanova V. K., Derckacheva N. I., Mankin A. S. Unusual nucleotide sequence heterogeneity of small multicopy pHSB plasmid from Halobacterium strain SB3, an archaebacterium. Can J Microbiol. 1989 Jan;35(1):160–163. doi: 10.1139/m89-024. [DOI] [PubMed] [Google Scholar]
- Lam W. L., Doolittle W. F. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5478–5482. doi: 10.1073/pnas.86.14.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Garrett R. A. Chloramphenicol resistance mutations in the single 23S rRNA gene of the archaeon Halobacterium halobium. J Bacteriol. 1991 Jun;173(11):3559–3563. doi: 10.1128/jb.173.11.3559-3563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Kagramanova V. K. Complex promoter pattern of the single ribosomal RNA operon of an archaebacterium Halobacterium halobium. Nucleic Acids Res. 1988 May 25;16(10):4679–4692. doi: 10.1093/nar/16.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Teterina N. L., Rubtsov P. M., Baratova L. A., Kagramanova V. K. Putative promoter region of rRNA operon from archaebacterium Halobacterium halobium. Nucleic Acids Res. 1984 Aug 24;12(16):6537–6546. doi: 10.1093/nar/12.16.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Mougey E. B. News from the nucleolus: rRNA gene expression. Trends Biochem Sci. 1991 Feb;16(2):58–62. doi: 10.1016/0968-0004(91)90025-q. [DOI] [PubMed] [Google Scholar]
- Sweeney R., Yao M. C. Identifying functional regions of rRNA by insertion mutagenesis and complete gene replacement in Tetrahymena thermophila. EMBO J. 1989 Mar;8(3):933–938. doi: 10.1002/j.1460-2075.1989.tb03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]