Abstract
The National Institute for Health and Clinical Excellence (NICE) guidelines for the management of atrial fibrillation were published in June 2006. It was anticipated that they would potentially lead to increased demand for echocardiography (ECHO), increased access to secondary care services (for example for cardioversion), and require additional resources for monitoring anticoagulation. A primary care survey was therefore initiated in South Devon, in advance of publication of the guidelines as a snapshot of existing practice, to determine any additional resources and education required to meet the new standards. The main aim was to determine what proportion of patients were managed exclusively in primary care, how frequently patients were investigated by ECHO and whether anticoagulation was being appropriately targeted at patients at high risk of thromboembolic events.
Key Words: anticoagulation, atrial fibrillation, echocardiography, National Institute for Health and Clinical Excellence guidelines, primary care
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. Its prevalence and incidence is believed to be increasing because of population ageing and increased survival from chronic conditions that predispose to having the condition.1 Several UK studies have demonstrated this trend.2,3 Atrial fibrillation is associated with increased morbidity and mortality particularly from thromboembolic stroke.4–6 Anticoagulation has been shown to reduce this risk and is an essential component of management, irrespective of whether a strategy of ‘rate control’ or ‘rhythm control’ is adopted.7,8
Despite established principles for the management of AF, it has been recognised that there is considerable variation in the approach to investigation and treatment of these patients.9 In an effort to address this, new guidelines for the management of AF were published by the National Institute for Health and Clinical Excellence (NICE) in June 2006.10
It was anticipated that the new guidelines would potentially lead to increased demand for echocardiography (ECHO), increased access to secondary care services (for example for cardioversion) and require additional resources for monitoring anticoagulation. A survey was therefore initiated in South Devon, in advance of publication of the guidelines as a snapshot of existing practice, to determine any additional resources and education required to meet the new standards.
South Devon has a mixed urban and rural population with a high proportion of elderly patients. Well-established open access ECHO services are available to local general practitioners (GPs), and many practices have access to cardiac monitoring independent of secondary care services. The main aim was to determine what proportion of patients were managed exclusively in primary care, how frequently patients were investigated by ECHO and whether anticoagulation was being appropriately targeted at patients at high risk of thromboembolic events.
Methods
All GP practices in South Devon were invited to take part in a systematic audit of a random sample of patients with AF during 2006. Participating practices made practice registers and records available for access by a specialist cardiac audit nurse. For each participating practice four patients were selected at random from the register of patients with AF for detailed review of case notes and source documentation. The audit addressed documentation of AF, investigations conducted, associated comorbidities (particularly in relation to cerebrovascular event (CVE) risk), thromboembolism prophylaxis and whether patients were referred to secondary care. Results were analysed using the paired t test (Strata v9.2 for windows) and a p value of 0.05 was considered statistically significant.
The audit nurse extracted relevant data from primary care case notes according to a predetermined template and arranged for all electrocardiograms (ECGs) and cardiac monitor data to be reviewed by a cardiologist for verification. Atrial fibrillation was classified as paroxysmal if it was self terminating and lasted less than seven days, persistent if more than seven days and permanent if chronic and long standing where cardioversion failed or not attempted.
Out of the 35 practices contacted, 33 agreed to take part. These practices serve a total population of 244,367 patients of whom 4,340 were recorded as having AF, giving an overall prevalence of 1.7%. The sample population selected for detailed study was 131 patients (no available data for one patient).
Results
Demographics
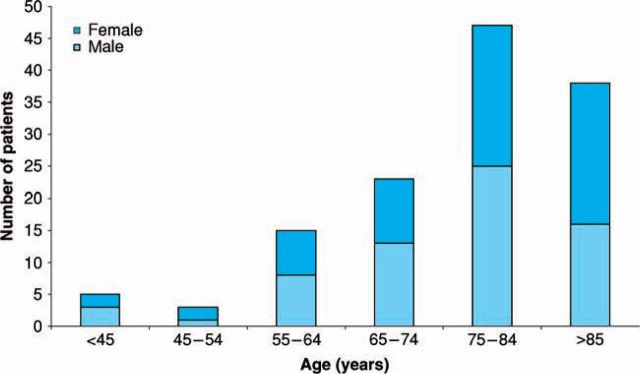
In the study population of 131, 66 patients were male and the mean age was 75 years (±13 years). The age distribution of patients within the study population is shown in Figure 1.
Fig 1.
Age distribution of patients with atrial fibrillation in primary care.
Diagnosis
Seventy three (56%) patients had either a standard 12-lead ECG or a cardiac monitor result within their primary care record documenting AF. In none of these cases was the diagnosis inaccurate. Of the remainder, a further 28% of patients had correspondence from secondary care recording AF. In 16% of patients no clear confirmation of the accuracy of the original diagnosis was available. In total, 60% of patients had permanent AF, 35% had paroxysmal AF (PAF) and 5% had persistent AF.
Investigations and onward referral
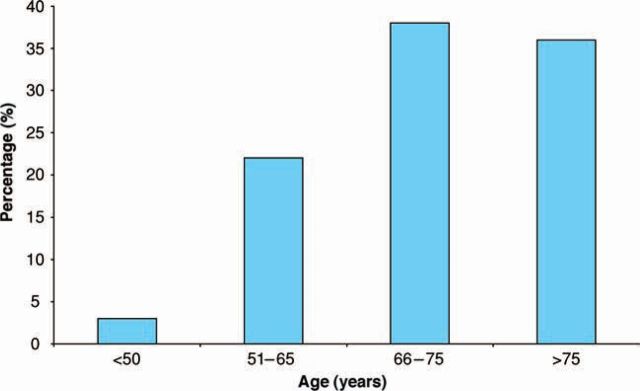
A total of 58 (44%) patients had documentation of an ECHO and 43 of these were older than 65 years of age. The age distribution of patients who had an ECHO is shown in Figure 2. Of patients managed exclusively in primary care, 18% had a documented ECHO result compared to 72% of those referred to cardiology (p<0.001). Of patients diagnosed since 2000, 57% had ECHO compared with 18% of patients diagnosed before 2000 (p<0.001).
Fig 2.
Percentage of patients who received an echocardiogram (ECHO) by age group.
In total, 68 patients (52%) had been referred to secondary care of which 22 (17%) had undergone at least one cardioversion. Of patients having an ECHO, 26% were recorded as having impaired left ventricular contraction (ejection fraction (EF) <50%). Of these 33% were in the cohort classified on clinical grounds (CHADS2) as intermediate/low risk. In addition, 31% of low/intermediate patients had a dilated left atrium (LA) (>45 mm).
Associated comorbidities
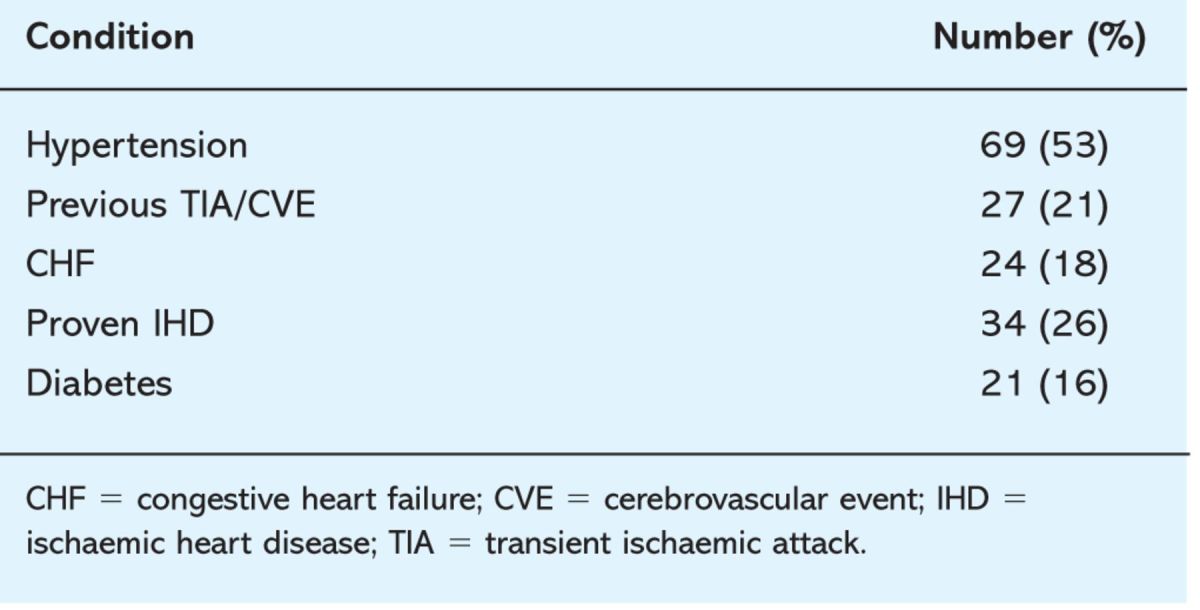
As might be expected in a cohort of this age, comorbidities were common. Of the patients studied, 21% had already had one or more transient ischaemic attack (TIA) or stroke. Other comorbidities are listed in Table 1.
Table 1.
Associated conditions in patients with atrial fibrillation.
Anticoagulation
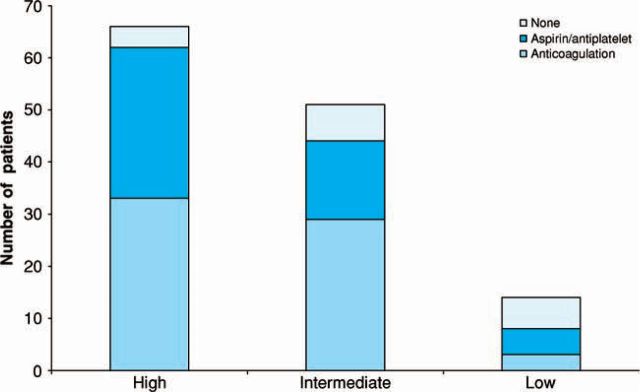
Of the study population 85% of patients were taking either aspirin or warfarin. In total, 64 (49%) were on warfarin and 48 (37%) on aspirin. According to NICE thromboembolic risk guidelines, 66 (50%) were high, 51 (39%) intermediate and 14 (11%) low risk. The number of patients in each group and treatment received are summarised in Figure 3. Of the patients on warfarin, 52% were female and 59% were over 75 years of age. Of those with a prior history of CVE/TIA, 61% were on warfarin.
Fig 3.
The number of patients in each group and the treatment received according to the National Institute for Health and Clinical Excellence thromboembolic risk guidelines.
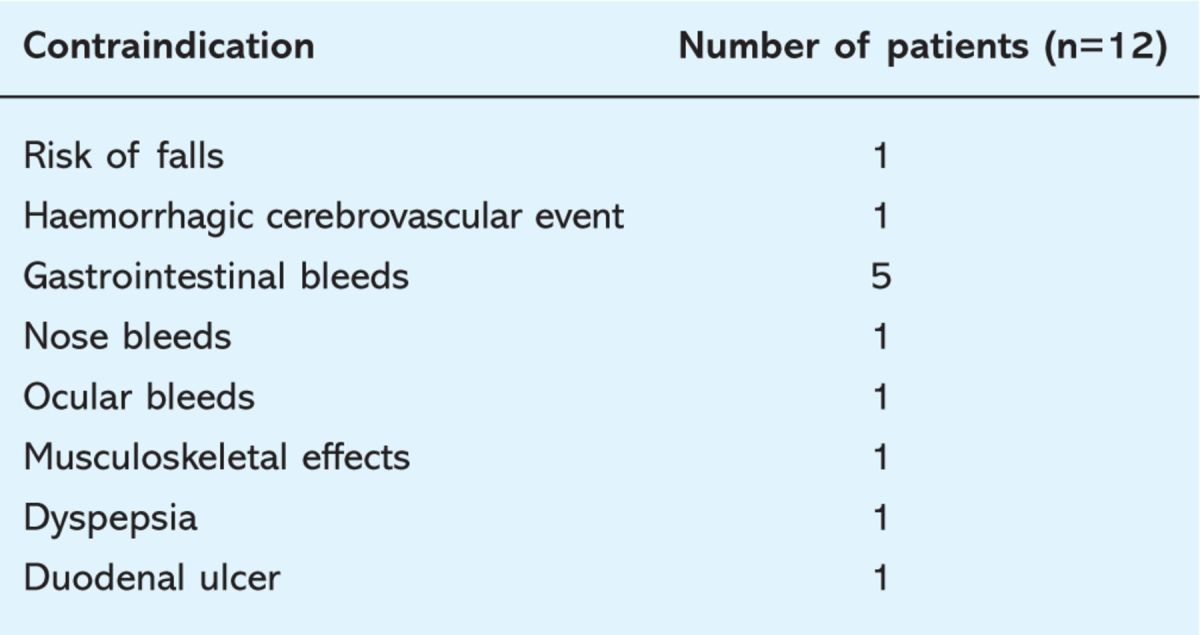
Twelve patients had contraindications to warfarin/antiplatelet therapy, of which six were in the high-risk group. The contraindications are listed in Table 2. Four patients refused treatment. The number of patients that were on no treatment, that did not have a specific contraindication or did not refuse treatment were three in the high (4%), four in the intermediate (8%) and three (21%) in the low-risk groups.
Table 2.
Contraindications to anticoagulation among patients with atrial fibrillation.
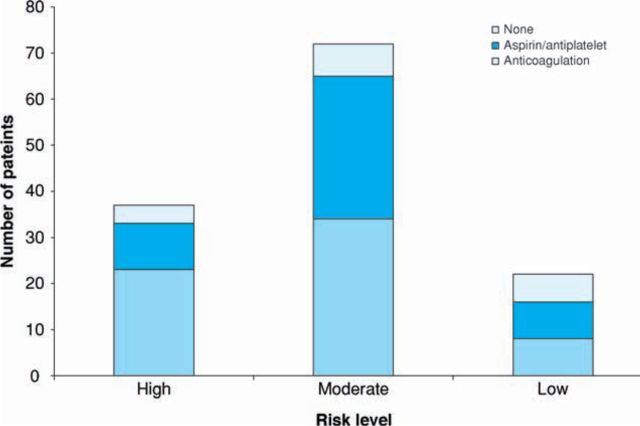
When the CHADS2 score for predicting stroke risk was used, 37 patients (28%) were high risk, 72 (55%) were moderate and 22 (18%) were low risk.11 Details of numbers receiving anticoagulation, antiplatelets or no treatment in the three groups are shown in Figure 4.
Fig 4.
The number of patients in each group and the treatment received according CHADS2 risk guidelines.
Discussion
The prevalence of AF in this study in all groups and sexes was 1.7%. Other UK studies have also reported its prevalence. A study of 4,522 patients’ ≥50 years of age in two GP practices in West Birmingham showed that 2.4% had arrhythmia.12 The prevalence of read-coded AF in 131 GPs in the DIN-LINK database increased from 0.84% to 1.49% in men and from 0.83% to 1.29% in women between 1994 to 2003.13 Data from the General Practice Research Database showed an overall prevalence of around 1%.14 The slightly higher prevalence rate in this survey probably relates to the level of elderly population in the catchment area.
Previous studies have alluded to the fact that ECG documentation of AF is important. In the cardiovascular heart study, 12% of AF cases were identified by ECG screening. In this survey 57% of patients had ECG validation in the practice register. In total, 84% of patients had either ECG documentation in primary care and/or a secondary care diagnosis of AF. Also, 44% of patients had an ECHO. This is slightly higher than in previous studies. The West Birmingham study reported that only around 20% of patients had an ECHO. It was also noted that the trend favoured patients who had a diagnosis after 2000 with 57% having had one compared to 18% in those diagnosed before 2000. A higher proportion of ECHOs were also performed if there was cardiology input. These findings may represent better availability of this procedure in general and also a lower threshold for GPs to request them in light of awareness of heart failure diagnosis or education regarding AF. However, it was still surprising that only 72% of patients had an ECHO if cardiology was involved. Previous guidelines have encouraged the procedure in AF patients.9 The less proscriptive nature of NICE guidance, which was not anticipated at the time of this study, recognises that thromboembolic risk stratification often does not require an ECHO. Nevertheless this procedure may be valuable for detecting adjunctive pathology and, as the results show, in the low/intermediate risk groups about 32% had some marker of increased stroke risk with an ECHO.
The overall rate of anticoagulation was higher than the 23% found by Sudlow et al., and the 31% by Karla et al.15,16 The Scottish Continuous Morbidity Recording Service database demonstrated that only 40% of patients were anticoagulated. In the study by Majeed and colleagues the rate of anticoagulation was around 30%.14
In this study over half the patients prescribed warfarin were female and aged over 75 years old. According to NICE guidelines for thromboembolic risk, 66 patients were high, 51 were intermediate and 14 were low risk. Although overall anticoagulation rates are higher than in previous studies, there is still discrepancy in the groups with only 50% of patients in the high-risk group on warfarin while in the low-risk group 21% were on warfarin.
When comparing the two risk scores used, the NICE guidelines stratified a larger proportion of patients into the high- and intermediate-risk groups, whereas with CHADS2 there were more patients in the low-risk group and the majority of the patients were in the intermediate-risk group. This reflects the different age thresholds in the NICE and CHADS2 guidance (65 years and 75 years respectively). The results of this study show that actual thromboembolic treatment choices conformed more closely to NICE, rather than CHADS2, guidance.
The high number of patients identified with a ‘moderate’ thromboembolic risk emphasises an important limitation of the guidelines in providing decision support for choice of antithrombotic regimen. NICE guidance suggests that warfarin or aspirin can be chosen in patients at intermediate risk. This could be interpreted as suggesting equivalence of these treatments, under which circumstance there would be little incentive to prescribe warfarin. However for many patients within this cohort warfarin may have significant advantages. For example, in the study by Mant et al, patients above 75 years old who were on warfarin had a significantly lower rate of stroke than those on aspirin without an excess risk of extra cranial bleeding.17 Furthermore, a patient having stroke as a single risk factor has a CHADS2 score of 2, which is classed as moderate risk but in reality is high risk. Therefore, classification of a patient at intermediate risk really serves only to invite more detailed evaluation, but inconsistency in the weight afforded to risk factors between guidelines can lead to continued uncertainty over appropriate treatment choices in this group.
Limitations
This was a retrospective study from GP registers with a moderate sample size which may not have captured all patients. However the relatively high prevalence of AF compared with other studies suggests that the majority of patients are listed on the registers. Although 50% of patients had ECG validation of AF, a proportion remains where there was no documentary evidence of AF. These patients are less likely to have been investigated and treated with anticoagulants so some overestimation of appropriate therapeutic intervention may have occurred.
Conclusions
In this study almost half of the patients with AF were managed in primary care alone. Electrocardiogram diagnosis in primary care was accurate, but these patients were less likely to undergo further investigation by ECHO. Patients diagnosed more recently are more likely to have had this latter procedure which may reflect greater awareness of the utility of this investigation or better access. Rates of anticoagulation are higher than in previous studies but there remains scope for better use of risk stratification algorithms to target thromboembolism prophylaxis appropriately. The cohort of patients for whom the NICE guidelines equivocate over thromboembolism prophylaxis is substantial.
Acknowledgements
We would like to thank all the primary care practices that participated in the study and also the Peninsula Cardiac Network who provided the funding for this study.
Reference
- 1.MacIntyre K, Capewell S, Stewart S. et al Evidence of improving prognosis in heart failure: trends in case fatalities in 66547 hospitalised patients between 1986 and 1995. Circulation 2000; 102:1126–31. [DOI] [PubMed] [Google Scholar]
- 2.Murphy NF, Simpson CS, Jhund PS. et al A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart 2007; 93:606–12. 10.1136/hrt.2006.107573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitmaurice DA, Hobbs FD, Jowett S. et al Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomized controlled trial. BMJ 2007; 335:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin HJ, Wolf PA, Kelly Hayes M. et al Stroke severity in atrial fibrillation: The Framingham study. Stroke 1996; 10:1765–9. 10.1161/01.STR.27.10.1760 [DOI] [PubMed] [Google Scholar]
- 5.Marini C, De Santis F, Sacco S. et al Contribution of atrial fibrillation to incidence and outcome of ischaemic stroke: results from a population based study. Stroke 2005; 36:1115–9. 10.1161/01.STR.0000166053.83476.4a [DOI] [PubMed] [Google Scholar]
- 6.Tsang TS, Petty GW, Barnes ME. et al The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol 2003; 42:93–100. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder IC, Hagens VE, Boisker HA. et al A comparison of rate control and rhythm control in patients with recurrent persistent AF. N Engl J Med 2002; 347:1834–40. [DOI] [PubMed] [Google Scholar]
- 8.Wyse DG, Waldo AL, DiMarco JP. et al A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–33. [DOI] [PubMed] [Google Scholar]
- 9.Fuster V, Ryden LE, Cannom DS. et al for Task Force on Practice Guidelines, American College of Cardiology/American Heart Association; Committee for Practice Guidelines, European Society of Cardiology; European Heart Rhythm Association, Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation - full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice guidelines. Europace 2006; 8:651–754. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Atrial fibrillation: the management of atrial fibrillation. London: NICE, 2006. [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W. et al Validation of clinical classification schemes for predicting stroke. JAMA 2001; 285:2864–70. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Golding DJ, Nazir M. et al A survey of atrial fibrillation in general practice: the West Birmingham atrial fibrillation project. Br J Gen Practice 1997; 47:285–9. [PMC free article] [PubMed] [Google Scholar]
- 13.DeWilde S, Carey IM, Emmas C. et al Trends in the prevalence of diagnosed atrial fibrillation, and its treatment with anticoagulation and predictors of such treatment in UK primary care. Heart 2006; 92:1064–70. 10.1136/hrt.2005.069492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majeed A, Moser K, Carroll K. Trends in prevalence and management of AF in general practice in England and Wales 1994–1998, analysis of data from general practice research database. Heart 2001; 86:284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow CM, Rodgers H, Kenny KA. et al Service provision and use of anticoagulants in atrial fibrillation. BMJ 1995; 311:558–60. 10.1136/bmj.311.7004.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karla L, Yu G, Perez I. et al Prospective cohort study to determine if trial efficacy of anticoagulation for stroke prevention in atrial fibrillation translates into clinical effectiveness. BMJ 2000; 320:1236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mant J, Hobbs FD, Fletcher K. et al Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503. 10.1016/S0140-6736(07)61233-1 [DOI] [PubMed] [Google Scholar]