Introduction
The idea of replacing body parts has attracted interest for a long time. In the third century, legend holds that St Cosmas and St Damian succeeded in grafting a leg from a recently deceased Ethiopian to replace onto the body of a patient. Unfortunately, this has not proved to be repeatable – the fact that the laboratory assistants in many representations of this event have wings indicating that a celestial connection may be required to make the operation more likely to succeed.
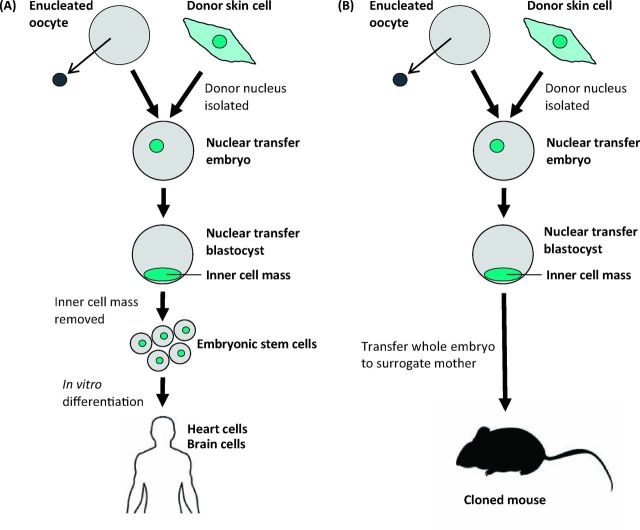
The more recent idea that it may be possible to take skin from a living person and, using the technique of cloning or nuclear transfer, make a replacement brain or heart is the focus of this Oration. Fig 1 illustrates the concept: the nucleus of a skin cell from the patient is transferred to an enucleated egg which forms an embryo. Embryonic stem cells (ESCs) can be derived from such an embryo and then directed into any tissue or organ desired. This theoretical scheme would give people new cells of their own genetic constitution, thereby avoiding problems of immunological rejection. This is an indication of the direction in which current research is moving at an encouraging pace.
Fig 1.
Single cell nuclear transfer for (A) therapeutic and (B) reproductive cloning.
The history of somatic cell nuclear transfer to eggs
Pioneering the principle: the work of Briggs and King
In the 1950s it was not known whether all cells of the body have the same genes. Subsequent work has shown that almost all cells of the body do in fact have the same genome. If this had not been the case, the production of replacement cells of one kind from available cells of an entirely different kind could never have been achieved. A reasonable proposition in the 1890s by August Weismann (1834–1914) was that, as cells differentiate during development, the new cell types form as a result of either the lack, or the permanent inactivation, of those genes no longer needed for a particular lineage.1 For example, skin cells might permanently lose the function of genes needed for the nervous system. In support of this concept, we now know that the process of cell differentiation is remarkably stable. Cells of one kind in the body almost never seem to switch directly into a completely unrelated cell type. Therefore an important aspect of the normal process of cell differentiation is that, as soon as a cell has embarked on a final type of differentiation, this status almost never changes.
However, this characteristic of stable cell differentiation can be reversed by ‘nuclear transfer’. The basis of this experimental procedure is to suck a cell into a fine micropipette such that the cell's wall is broken but the nucleus remains intact. This ruptured cell and intact nucleus is then injected into an unfertilised egg whose own chromosomes have been removed or destroyed by ultraviolet irradiation. This egg then begins development using the implanted nucleus in place of the egg and sperm nuclei which would normally be present after fertilisation. In some cases this transplanted nucleus in combination with the egg cytoplasm can develop into a normal adult animal (Fig 1). This procedure was pioneered by Briggs and King in 1952.2 However, the results they obtained at that time did not yield normal development from the nucleus of a specialised cell and therefore appeared to support the idea of Weissmann.
Early work with Xenopus
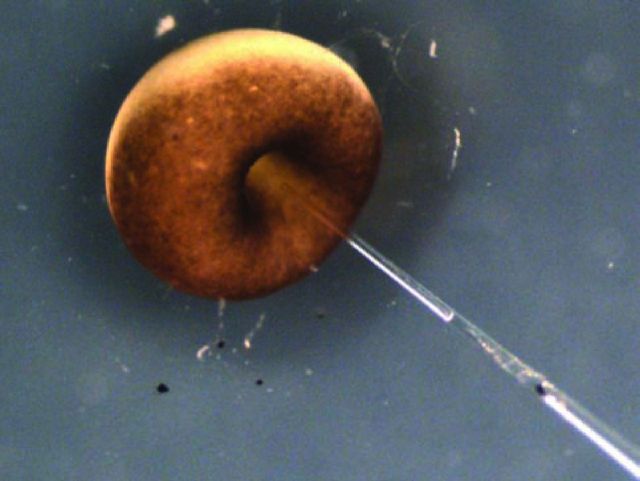
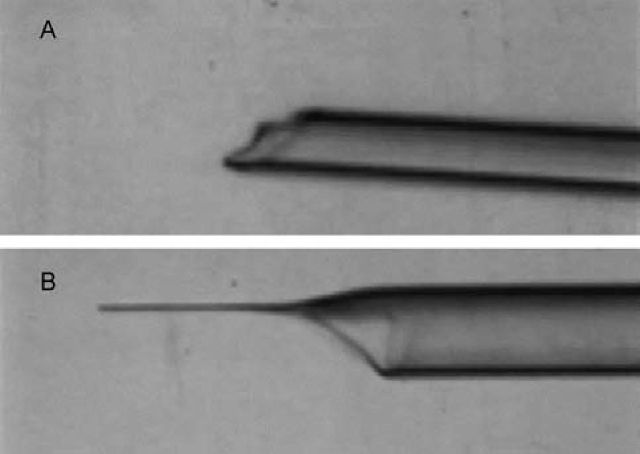
I was encouraged by my supervisor, Michail Fischberg, a lecturer at Oxford, to try to apply the technology developed by Briggs and King to the South African frog Xenopus. This was because Xenopus had enormous advantages for laboratory work over Rana pipiens, the species used by Briggs and King. Xenopus embryos can be grown to sexual maturity within a year and, being permanently aquatic, the animals can be kept in easily cleaned tanks of water rather than in a terrarium. But it was easier said than done. First, the Xenopus egg is surrounded by a thick layer of jelly that is totally impenetrable even with the sharpest of needles (Fig 2). By good luck my supervisor had recently bought a new ultra-violet microscope light source. We found that the ultra-violet emission of this source included a very low wavelength of light, which happened to dissolve the jelly of the Xenopus egg. Very conveniently, another wavelength of emission from this source destroyed DNA and therefore permitted the destruction of the chromosomes of the unfertilised egg. This UV-irradiation was much easier and more reliable than trying to remove the chromosomes in the unfertilised egg by micromanipulation. Even with the benefit of jelly depletion by ultraviolet light, it was necessary to make an extremely sharp needle with an unconstricted opening. Eventually a way was found of creating such a needle in glass, using a microforge that I made, so that it could gently rupture a cell but not the nucleus and yet contain a sharp enough point not to damage the recipient egg (Fig 3).
Fig 2.
Xenopus egg. The egg is surrounded by a thick layer of jelly that is impenetrable by needles.
Fig 3.
Microinjection needles (A) before microforging (B) after microforging.
Another piece of good fortune resulted from the wisdom of my supervisor. He had a student whose project involved studying the nucleolus of these amphibian cells. The project involved killing the egg nucleus of a fertilised egg to create haploid embryos, recognisable by having only one nucleolus in their cells (diploid embryos have two nucleoli per nucleus); haploid embryos always die before becoming a swimming tadpole. The student reported that she had obtained one-nucleolated embryos which survived entirely normally. Wisely my supervisor said to the student, ‘you must find the actual frog which gave you this extraordinary result.’ Very surprisingly, the frog concerned was a natural mutation in which all its cells had a single nucleolus instead of the normal two for a diploid embryo; it had lost all ribosomal genes on one chromosome set. The really important point was that this immediately provided an invaluable genetic marker for nuclear transfer experiments. Without this, the experiments I did would probably have never been believed.
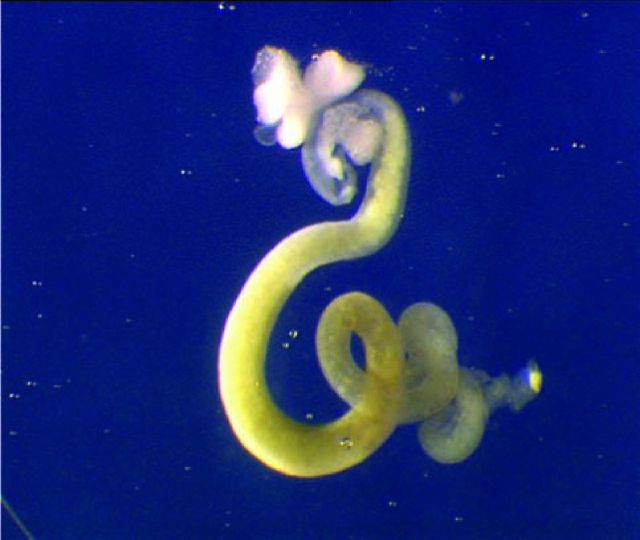
Within a couple of years of starting graduate work, I was fortunate that I had been able to obtain entirely normal embryo development from the transplanted nucleus of a feeding tadpole's intestinal epithelium (Fig 4). In the course of time, some of these embryos developed into sexually mature adult animals. They therefore proved that the nucleus of an intestine cell can form every cell type in the body and therefore that the process of differentiation cannot involve the loss or permanent inactivation of any genes.3 This conclusion differed from that of Briggs and King and, thanks to the use of the genetic nuclear marker, was in time accepted by the scientific community.
Fig 4.
Intestinal tract of feeding tadpole (GFP marked).
From amphibians to mammals
In the early 1960s, a journalist asked me whether this kind of work could ever succeed in mammals or even in humans. I said that I had no idea, but guessed that anything from 10–100 years might be necessary for this to be achieved. It turned out to be a good guess because it was actually 45 years later that the first successful somatic cell nuclear transplantation to mammalian eggs was reported, resulting in Dolly the sheep. In the end, the experimental design used to create Dolly was the same as that used for the Xenopus frog work. The problem was that many of those who had tried to repeat the amphibian experiments with mammals failed to use exactly the same procedure.
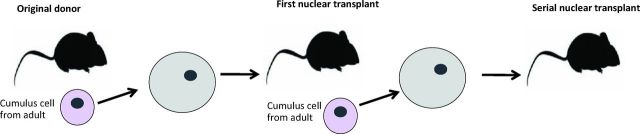
Nowadays we know that most kinds of mammals can be cloned using somatic cell nuclear transfer to eggs. Each species has particular characteristics often requiring a large amount of work to discover ways of overcoming these obstacles. Recently there has been success in nuclear transfer in humans, though it is not permitted to grow the embryos obtained into advanced embryos or fetuses. In mice, this nuclear transfer procedure has been refined to an extraordinary extent. Dr Wakayama, in Japan, has even succeeded in carrying out a serial nuclear transfer experiment.4 He transplanted the nucleus of a cumulus cell to a mouse egg and grew that embryo to an adult. He then took a cumulus cell from that mouse and transplanted its nucleus back to another egg. In the end, he did this 25 times. Therefore the original transplanted nucleus had undergone some 500 cell divisions, much more than any cell would ever do in the whole life of a mouse or human. Nevertheless, that nucleus retained its totipotentiality and never underwent any kind of death or became cancerous (Fig 5). In this way the egg can be seen to immortalise the nucleus of a specialised cell, and therefore provide a source of rejuvenated embryo cells indefinitely.
Fig 5.
Serial nuclear transplantation. Wakayama et al (2013)4 demonstrated 25 generations of serial nuclear transfer, with 500 cell divisions from the first donor nucleus. No cancer incidence was observed.
The work discussed above leads to two fundamental conclusions. One is that as cells undergo normal differentiation from an embryo to an adult, almost all cells retain a complete and intact genome; no genes are lost or permanently inactivated. Second, an egg has the ability to rejuvenate the nucleus of a specialised cell so that it can create cells which have gone back in development to an embryonic state and can therefore, in principle, be a source of rejuvenated cells for cell replacement.
Basic principles of cell differentiation
Having said that nuclear transfer to eggs can fully reprogramme the nucleus of an adult cell, this does not always happen. In fact, when starting from a specialised cell, the success with which the transplanted nucleus from that cell can, in combination with an enucleated egg, form an entirely normal, sexually mature individual is only about 1 or 2%. Most embryos formed in this kind of experiment develop abnormally and do not reach the adult stage. The question therefore arises as to how, in normal development, new kinds of cells arise, all starting from a single, fertilised egg cell.
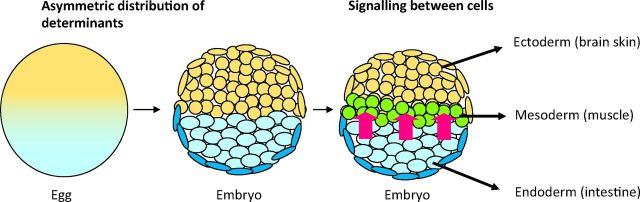
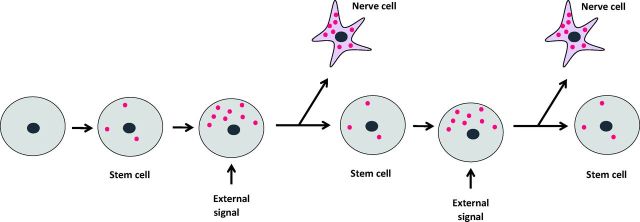
There are two fundamental principles by which this is achieved (Fig 6). The first principle is that as a fertilised egg divides into its daughter cells, some of the components of the egg are asymmetrically distributed to the two daughter cells, causing the daughter cells and their progeny to specialise in different directions. This process of the asymmetric distribution of parental cell components continues through much of life. This is particularly clear in the case of the invertebrate nervous system. Typically, a parental mother cell divides into two unequally sized daughters. The smaller daughter acquires a very high concentration of components from one part of the mother cell. The other larger daughter has a much lower concentration of these components. As a result the small cell with the high concentration of components specialises into a mature nerve cell. The other cell typically remains as a ‘stem cell’ and then makes further asymmetric divisions (Fig 7).
Fig 6.
Mechanisms of cell differentiation. The first mechanism is asymmetric distribution of determinants in the egg, giving rise to an asymmetric distribution of cells in the embryo. Subsequently, signalling between cells (red arrows) causes further differentiation to give the ectoderm, mesoderm and endoderm.
Fig 7.
Formation of specialised cells from stem cells. Gene activation molecules accumulate in the stem cell and move to one side in response to an external signal. Asymmetric cell division gives rise to a specialised skin cell and depends on the concentration of transcription factors (red spots).
The other principle is that cells of one kind frequently signal to cells of another. They release protein factors which activate selected genes. These factors, often referred to as growth factors, cause the cells that receive them to change their course of differentiation and make a new cell type. Signalling in this way is a very common characteristic of normal embryo development.
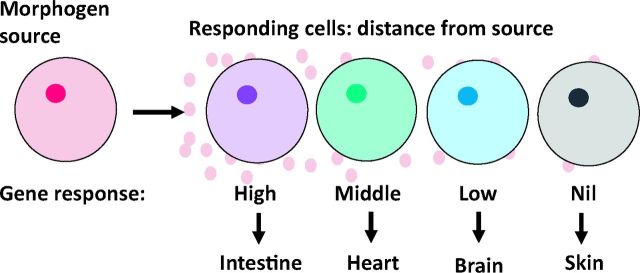
Signalling from one cell to another often takes place in a concentration-dependent way. The signal factors released by one cell become diluted as they spread through an increasingly distant set of receiving cells. Importantly, it is the concentration of these factors that ultimately decides the fate of the responding cells. Those receiving a high concentration will differentiate in one direction, for example becoming muscle, whereas those receiving a lower concentration at the same time will specialise in another way, becoming skin or brain. This principle of a morphogen gradient commonly accompanies signalling and so increases the range of cell types that can be formed by a single signalling event (Fig 8).
Fig 8.
Cells specialise in ways determined by the concentration of morphogen molecules (pink circles) that come from the source and reach distant cells by diffusion.
As a result of these two processes acting in combination, the whole range of specialised cell types is formed. Once formed, as has been said, the specialised state of these cells continues for life.
A further variant of signalling between cells has been discovered: in what is termed the ‘community effect’, the absolute concentration of signalling factor can be built up so long as a large number of cells each contribute to it. These cells have to be in close proximity to each other. Distant cells separated by many other non-contributing cells would never provide a sufficient concentration of signalling factor to be effective. This mechanism seems to be important when a large number of cells need to differentiate in the same direction. Interestingly, the same basic principle, ‘quorum sensing’, was discovered in bacteria which create fluorescent light in the light organ of certain fish. Only when the bacteria achieve a sufficiently high concentration do they cause the light organ to emit light and hence act as a bait to attract freshwater animals so that they can serve as a source of food for the fish. It is interesting that the same fundamental principle can emerge from two completely unrelated kinds of work.
The mechanisms behind nuclear transfer
Meiotic oocytes and nuclear reprogramming
It generally turns out that if a new procedure achieves something useful it is valuable to understand its mechanism. Thus it would be scientifically interesting and of potential practical value to identify the reprogramming components of an amphibian egg. An obvious idea is to separate out the components of eggs and see whether they can reprogramme nuclei in a test tube. However, this has never proved a successful approach to studying mechanisms of gene transcription. It therefore seemed worth trying a lateral approach, as exemplified by the story of the discovery of nerve growth factor by Cohen and Levi-Montalcini, who received their Nobel Prize for this work in 1986.5 They had been struggling for a long time to analyse the material in a hen's egg that can induce massive nerve growth, using a phosphodisesterase enzyme obtained from a very rare moccasin snake. However, on discovering that the world's supply of moccasin snakes was insufficient for them to finish their experiments, they had the clever idea that snake venom might be closely related to the products of salivary glands of mice and rats. So they forgot about moccasin snakes and worked with rat salivary glands, which also seemed to have phosphodiesterase activity, and succeeded in identifying the nerve growth factor. The principle here seems to be that if you are struggling with difficult material it always pays to see if some readily obtainable alternative will work as well.
We followed this principle in trying to identify the reprogramming components of eggs. We were hampered by the fact that most somatic nuclei, which divide very slowly, undergo extensive chromosome loss and damage when transplanted to eggs due to the very fast rate of replication imposed by the egg on somatic nuclei. We needed an assay which tested transcription (RNA synthesis) rather than DNA replication, and chose to test the growing oocyte of an ovary in first meiotic prophase as an alternative to an egg. We found that we could inject multiple somatic nuclei into the specialised nucleus (germinal vesicle) of the oocyte, which is a natural egg progenitor. Remarkably, the injected nuclei were rapidly reprogrammed to start expressing pluripotency genes which are not expressed in adult cells. This effect was seen in a very high percentage of injected nuclei. Furthermore, we found that the somatic nuclei of mammalian cells worked just as well as those of frog cells. The so-called germinal vesicle of an oocyte has a very high concentration of developmentally important components, since the germinal vesicle contents are normally dispersed into the naturally fertilised egg and are necessary for normal development. This gave us an invaluable test system to identify the reprogramming components of an egg that are directly derived from the germinal vesicle of an oocyte.
It is interesting that if you incubate somatic nuclei in extracts of eggs or oocytes they do not undergo any significant transcription. If, however, you inject multiple nuclei into a living oocyte, transcription is re-initiated several hundred times in a day. This gives an invaluable test system for factors affecting transcription and reprogramming. A key characteristic of an oocyte is that, unlike an egg, it never induces DNA replication of transplanted nuclei, and so causes no chromosome damage.
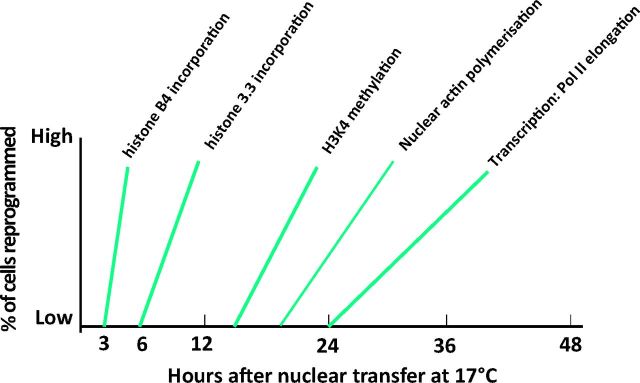
As a result of numerous experiments, we have some idea of the sequence of events that leads to the reprogramming of a transplanted somatic nucleus. As seen in Fig 9, the first crucial event is an invasion of the transplanted somatic nuclei by a specialised oocyte-specific linker histone. This happens within a few hours of nuclear transfer and is a necessary step towards the induced transcription of pluripotency stem-cell-like genes. Soon after this, another histone variant, namely H3.3, displaces the linker histone and opens up the previously quiescent genes for transcription by RNA polymerase II. There are no doubt many other steps to be discovered. But the general principle seems to be that the oocyte is endowed with a very high concentration of the components that can turn a somatic nucleus back from its adult state into an embryonic one. These same components are almost certainly active in the newly fertilised egg when they have to decondense and activate (reprogramme) the highly specialised sperm nucleus after fertilisation.
Fig 9.
Time course of transcriptional activation of transplanted somatic cell nuclei by oocytes.
Overcoming resistance to reprogramming
The next major question in this field asks why the reprogramming components of an egg do not work successfully in all somatic nuclei transplanted to them. This is evidently due to a strong resistance of the somatic nucleus towards being reprogrammed by an egg. As we have said, somatic cells are extremely stable in their differentiation and would never normally be reprogrammed by an egg in the way that a sperm is. The egg does a rather good job of reprogramming many of the quiescent genes introduced with a somatic nucleus but cannot do it perfectly. We need to know the basis of this resistance and hence stability of differentiation. Using the oocyte assay we can progressively remove components from the transplanted nuclei to find out which components are important in giving this stable resistance to reprogramming.
We now know some of the most important stabilising components. One of these is the methylation of DNA in a somatic nucleus; the nucleus strongly resists demethylation by an oocyte. Another key factor is modification of the histones that are wrapped up with the DNA in somatic nucleosomes. An example of this is histone H2A lysine 27 trimethylation. In other cases, we find that a combination of several different histone modifications is required. Gradually we are discovering which histone modifications are important (such as the ubiquitination of histone H2A) and how they can resist reprogramming, often in combination. Looking further ahead, it seems that the spacing of nucleosomes between genes may also be so arranged that it is difficult for egg cytoplasmic components to rearrange these. In the long term it should be possible to identify most or even all of the resistance-inducing components of a somatic nucleus and then remove them or cancel their effect. In the end this might make it possible to reprogramme somatic nuclei with very high efficiency. This could prove particularly valuable when we discuss the spectacular advances now made in the induction of pluripotency of stem cells.
Induced pluripotency
Some of the great discoveries in biomedical science have come completely unexpectedly. No one would have predicted the discovery, in 1981, by Martin Evans that permanent cultures of embryonic cells could be made.6 Evans discovered that certain cells taken from a strain of mice could be placed in culture under specified conditions and that these cells would proliferate indefinitely without proceeding, as embryonic cells usually do, to various kinds of differentiation. Even more impressively, under particular culture conditions these cells could be induced to differentiate into specialised cells of desired kinds.
As long ago as the 1980s, Weintraub and colleagues were able, through an ingenious design, to isolate a particular transcription factor which they named MyoD. They then overexpressed this protein in a range of different kinds of cells and found, remarkably, that in many cases this factor could induce cells to become muscle, even if the cells were not muscle related in their differentiation state.7 This established the fundamental principle that the massive overexpression of a new factor can, in many cases, transform a cell, or at least some of its daughter cells, so that they follow a pathway of differentiation related to that of the transcription factor.
For a long time MyoD was the only demonstrated example of this principle. However, in 2006, Takahashi and Yamanaka announced the amazing finding that the overexpression of a selection of transcription factors, specifically those characteristic of embryonic cells, could make an adult cell go back to an embryonic state.8 The success rate was extremely small, being about one in 10,000 cells or less. Nevertheless, if this one cell was selected and grown in the laboratory, it and its daughters had the characteristics of an ESC. It was almost immediately seen that this finding had enormous possibilities. First, it would be possible to take cells from an adult animal or human and derive from them cells having the characteristics of ESCs as described above. Second, it was realised that if it is possible to derive growing cells from the diseased cells of a patient, these cells could be cultured in the laboratory and tested for any number of drugs that might arrest or even reverse the diseased state. Since it is not ethical to test new drugs on living humans, this would make it possible to test drugs with cultured somatic cells in the laboratory and so screen them for their potential value in therapeutic treatment.
The discovery of Takahashi and Yamanaka precipitated a tidal wave of activity in this field. Once the technique had been demonstrated, it proved very simple to grow these cells and to try to improve the efficiency with which the induced pluripotency worked. In fact, there must now be thousands of institutes across the world set up under the heading of ‘regenerative medicine’ using the induced pluripotency procedure. As a result, the efficiency with which these ESC-like cells can be derived from animal and human tissues has improved. Nevertheless, the process is still surprisingly inefficient, since some 99% of cells, for some reason, do not respond to the overexpression of the known factors. It is actually rather surprising that the mechanism of action of this overexpression procedure is still obscure. That does not, however, prevent it from being extremely useful.
A great deal of work, particularly in the commercial sector, is going ahead to try to identify drugs that can alleviate human disorders, especially those involving neurodegeneration. The problem here is to know whether the cells in culture derived from a patient truly represent the disease. It could be that several different cell types have to undergo some change for the disease to affect a human; if so, it will be very difficult to truly represent the disease in culture. There is also concern that the cells derived by induced pluripotency may themselves not truly represent the disease; it is known that, the longer you culture cells in the laboratory, the greater the chance of genetic changes taking place. There are continuing efforts to try to characterise the derived pluripotency cells so that they accurately represent the desired cell type for replacement and can be safely transplanted to a patient. There is a view that the reprogramming induced by eggs, as described above, could ultimately be the best route. After all, the egg is provided with natural substances that are extremely effective in reprogramming sperm, a highly differentiated cell type. There is therefore a desire to understand how the egg reprogramming substances work, in order to use them in combination with induced pluripotency and provide the best possible cells for therapeutic replacement.
Replacement cell therapy
Recently there has been great success in obtaining cells that can alleviate the common human condition referred to as age-related macular degeneration. In this state, people gradually lose peripheral vision and eventually central vision, becoming blind, as a result of the death of cells called retinal pigmented epithelium cells. These cells provide essential support for the photoreceptor cells, absorbing harmful photoreceptor products. Retinal pigmented epithelium cells can be derived from human ESCs or indeed from human skin cells by induced pluripotency. Several companies and laboratories have been actively engaged in producing the required retinal pigmented epithelium cells. These cells can be grown in culture and then eventually plated as a monolayer on a thin plastic sheet. This sheet of cells can then be transplanted into the eye of a patient. It now seems that the replacement retinal pigmented epithelium cells can arrest the deterioration of vision and possibly even improve vision from a diseased state. The surgical treatment required is very mild; no more complicated than a cataract operation. When in full use, it should then be possible for a patient to go into an appropriate hospital or treatment centre and receive the replacement cells as an outpatient. Animal tests have been successful9,10 and permission has just been given for this treatment to be tested in humans in UK.
One might ask why this first therapeutic use of replacement cells should be applied to the eye. The answer seems to be that the absolute number of cells needed to provide new retinal pigmented epithelium is quite small, about 5×104 – far below what would be needed to replace part, for example, of a heart. A major heart infarction can kill over 1011 cells; it would be a forbidding task to grow this number of cells in a laboratory, so that they retain their desired characteristics and do not become cancerous. However, once the transplantation of retinal pigmented epithelium proves successful it could well be made available to the public at a cost comparable to that of cataract treatment. This cost would be considerably less than the annual cost of supporting a blind person to carry out their activities. It would be expensive to derive these retinal pigmented epithelium cells from each patient so that they are given back their own genetically compatible cells; however, the eye is an immunologically privileged site and it is possible that a patient could accept cells from a genetically unrelated source.
Wider issues
Regulatory bodies and lawyers
Before a new procedure can be tested on patients, most countries, including UK and USA, require that regulatory bodies approve the procedure. This is in order to avoid patients suffering from insufficiently tested new procedures. Unfortunately many such bodies have become extremely conservative and take an enormous time, and require extensive testing, before they give their approval for a procedure to be used on patients. In the particular case of the alleviation of macular degeneration of the eye, there has been massive delay in approval for this procedure to be tested on patients. When I mention this work to general audiences, I am amazed how many people say that they would love to have this procedure and, even if there is an element of risk, would willingly set aside their legal rights in order to have the opportunity of saving their vision from further deterioration. Even if, eventually, there was some problem with the treatment, the prospect of several extra years of life with vision is immensely attractive. I would very much hope that regulatory bodies can accelerate their procedures and approve procedures much more rapidly than at present.
A major problem that regulatory bodies have is that they are threatened by the ability of a judge to award punitive damages if the procedure is not wholly successful. Furthermore, I am told that the legal profession is extremely reluctant to allow a patient to set aside their legal rights on the grounds that the patient may not be fully informed about the procedure. I feel very strongly that a patient should be allowed to opt for a procedure, assuming that it has been explained to them, as far as possible, what the procedure involves and what risks might accompany the treatment, even if regulatory bodies have not finished their prolonged deliberations.
The value of basic research
It is interesting, now, to appreciate that this field of cell replacement could be seen to have originated in a basic science laboratory for purely scientific reasons. When decades ago we sought to find out whether all cells of the body have the same genome, there was no prospect at that time that this could have any therapeutic value. Most particularly, the discovery of permanently replicating ESCs was crucial to the idea that reprogrammed cells could be useful to a patient. Increasingly, we find that those applying for research grants have to state quite clearly in what respect the work they do will be beneficial to human health. Had this been the case when I started my work in the 1950s, the grant support I applied for might not have been given. The earliest frog nuclear transfer experiments were undertaken in 1952 and it was another 54 years before the human health relevance became clearly evident in 2006.
Most of us, as scientists, believe that any basic research project that is designed to answer a fundamental question in the field of cell biology and development is worth supporting, even if at the time its application is not evident.11
References
- 1 .Weismann A. Das Keimplasma, eine theorie der Vererbung. In: Parker WN, Ronnfeldt H. (eds), The germ-plasm: a theory of heredity. London: Walter Scott, 1892. [Google Scholar]
- 2 .Briggs R, King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc Nat Acad Sci 1952;38:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 .Gurdon JB, Uehlinger V. ‘Fertile’ intestine nuclei. Nature 1966;210:1240–1. [DOI] [PubMed] [Google Scholar]
- 4 .Wakayama S, Kohda T, Obokata H, et al. Successful serial recloning in the mouse over multiple generations. Cell Stem Cell 2013;12:293–7. [DOI] [PubMed] [Google Scholar]
- 5 .Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J 1987;6:1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 .Evans M. Origin of mouse embryonal carcinoma cells and the possibility of their direct isolation into tissue culture. J Reprod Fertil 1981;62:625–31. [DOI] [PubMed] [Google Scholar]
- 7 .Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987;51:987–1000. [DOI] [PubMed] [Google Scholar]
- 8 .Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 9 .Kamao H, Mandai M, Sakai N, Suga A, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep 2014;2:205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 .Kanemura H, Go MJ, Shikamura M, Nishishita N, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One 2014;9:e85336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 .Gurdon JB. From nuclear transfer to nuclear reprogramming: the reversal of cell differentiation. Ann Rev Cell Dev Biol 2006;22:1–22. [DOI] [PubMed] [Google Scholar]