ABSTRACT
Chronic hepatitis B (CHB) remains a global healthcare challenge, complicated by the development of cirrhosis and hepatocellular carcinoma, accounting for approximately 600,000 deaths per year. Hepatitis B is a DNA virus, which utilises a covalently closed circular (ccc) DNA to act as a transcriptional template for the virus. The persistence of cccDNA in the nucleus of infected hepatocytes accounts for HBV chronicity. Quantitative hepatitis B surface antigen (qHBsAg) acts as a surrogate for the level of cccDNA and therefore may provide useful information around treatment response and viral immune control. Current antiviral therapies are limited in their ability to achieve HBsAg loss, which is considered the ‘gold-standard’ treatment endpoint. This article focuses on the unmet needs in CHB today; a better definition of disease phase, the timing of therapeutic intervention, optimising treatment strategies with current therapies and the development of novel agents; all with HBsAg loss as the therapeutic goal.
KEYWORDS : cccDNA, pegylated interferon, nucleos(t)ide analogues, quantitative HBsAg, disease phase, liver biopsy, transient elastography, HCC
Key points
CHB is a complex dynamic disease requiring specialist input and management
CHB patients, both treated and untreated, require long-term monitoring
The utility of qHBsAg can enhance disease categorisation and guide management decisions
The limited efficacy of current antiviral therapies (PegIFN and NUCs) warrants the development of new therapeutic approaches
Future therapies should be individualised with the ultimate goal being eradication of virus.
Introduction
The emergence of directly acting antivirals for the treatment of hepatitis C virus (HCV) represents a realistic opportunity to eliminate hepatitis C; however, the lack of similar agents for the treatment of chronic hepatitis B virus (CHB) will mean renewed focus on the treatment and management of CHB in the coming years.
Despite the availability of a preventative vaccine for almost 40 years, the WHO estimates that more than 400 million people worldwide are chronically infected with hepatitis B virus (HBV). An estimated 600,000 deaths per year are attributed to cirrhosis and hepatocellular carcinoma (HCC) complicating CHB.1 In the UK, according to the National Institute of Health and Care Excellence (NICE), as many as 500,000 people may have chronic infection, with most disease seen among migrants who acquired their infection in childhood from their native country.2
HBV is a small enveloped DNA virus which infects hepatocytes. Within the hepatocyte the HBV genome is converted to a covalently closed circular (ccc) DNA that serves as a template for transcription of all the viral proteins.3 cccDNA plays a key role in the viral life cycle and its persistence in the nucleus of infected hepatocytes is the basis of HBV chronicity. Hepatitis B surface antigen (HBsAg), the hallmark of CHB, is thought to act as a surrogate for the level of cccDNA and thus recent studies have focused on the utility of quantitative HBsAg (qHBsAg) to profile disease and assess treatment response; with HBsAg loss considered the ‘gold-standard’ treatment endpoint.4
Current antiviral therapies include pegylated interferon (PegIFN) and nucleos(t)ide analogues (NUCs), both of which offer limited efficacy in achieving HBsAg loss, therefore life-long treatment with viral suppression represents the standard of care in the majority of patients. This underlines the challenges we face in the management of CHB and the need for novel treatment strategies to mirror the recent advances in the HCV treatment landscape.5
Natural history of HBV
Diagnosis
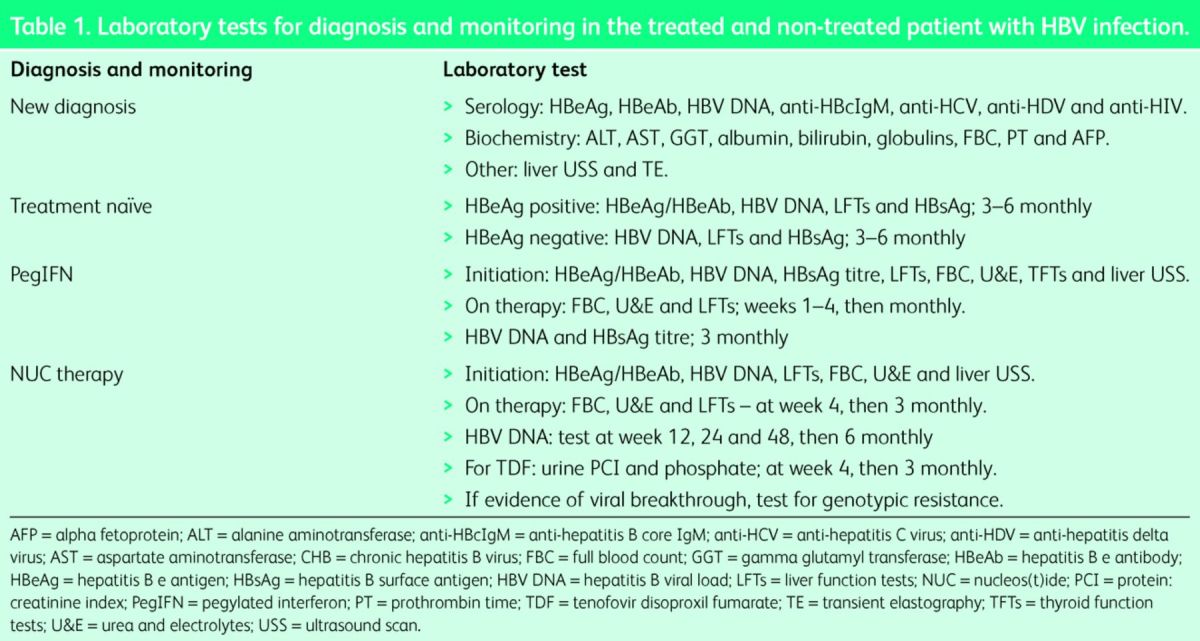
The detection of HBsAg in the serum on two occasions 6 months apart is diagnostic of CHB. However, HBsAg positivity should trigger a broader panel of investigations, including viral serology coupled with biochemical tests, to profile the disease and facilitate management (Table 1). Quantitative HBV DNA and the liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), are key markers of disease, reflecting replicating virus and liver inflammation/injury, respectively. In addition, a full chronic liver disease screen and baseline imaging should be performed for formal disease assessment (Table 1).
Table 1.
Laboratory tests for diagnosis and monitoring in the treated and non-treated patient with HBV infection.
Disease phase
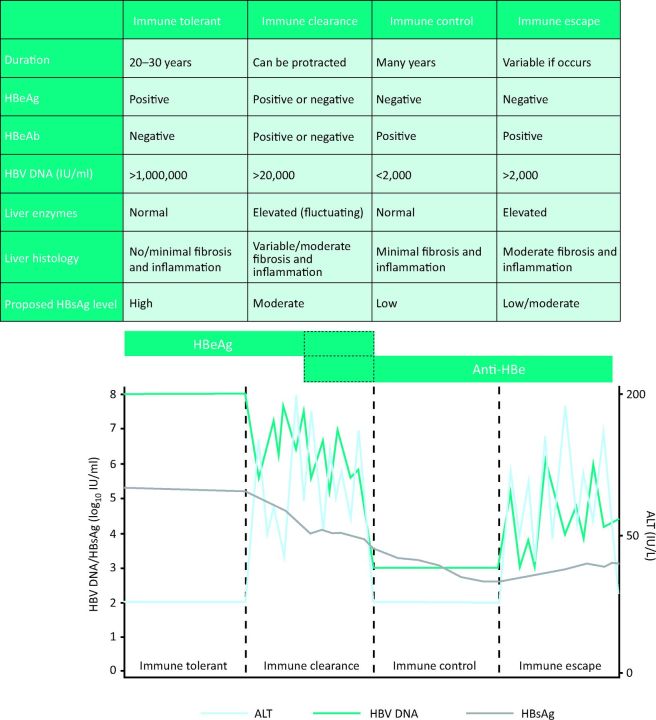
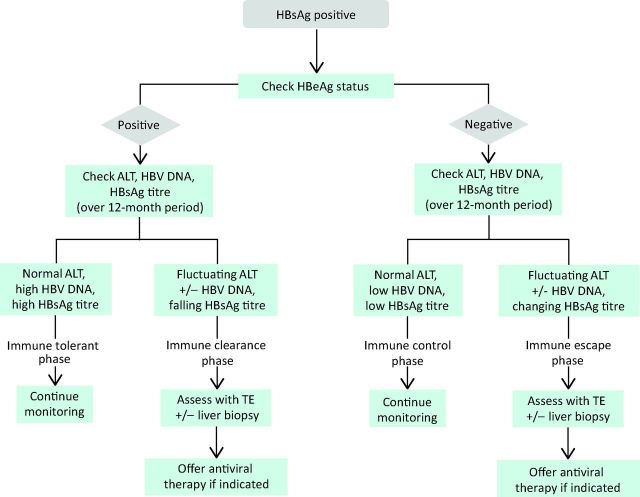
CHB is a dynamic disease characterised by four distinct disease phases or clinical categorisations, which are used to define disease state and determine management decisions (Fig 1 and 2).
Fig 1.
Characteristics of disease phase in CHB with corresponding schematic. ALT = alanine aminotransferase; CHB = chronic hepatitis B virus; HBeAg = hepatitis B e antigen; HBeAb = hepatitis B e antibody; HBsAg = hepatitis B surface antigen; HBV DNA = hepatitis B viral load.
Fig 2.
Algorithm for disease assessment and monitoring in each disease phase of CHB. ALT = alanine aminotransferase; CHB = chronic hepatitis B virus; HBeAg = hepatitis B e antigen; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; TE = transient elastography.
Immune tolerance is associated with perinatal infection or infection in early childhood and thus this phase is synonymous with disease in children or young people. The hallmarks of immune tolerance are hepatitis B e antigen (HBeAg) positivity, high levels of HBV DNA and normal serum ALT with minimal or no liver fibrosis on biopsy. This phase of disease is believed to last from a few years to several decades.6
The immune active (or immune clearance) phase is characterised by the presence of HBeAg, fluctuating levels of HBV DNA (usually >20,000 IU/ml) and elevated serum ALT, reflecting immune-mediated liver injury. HBeAg seroconversion during this phase may result in immune control of the virus often referred to as the ‘inactive carrier’ (IC) phase. Characterised by the loss of HBeAg, patients are anti-HBe positive with normal serum ALT and HBV DNA <2,000 IU/ml. This phase of disease represents the host's ability to control the virus; therefore transition from the immune active to the immune control phase is unpredictable and not an inevitable sequence of events.7
A proportion of inactive carriers may develop disease reactivation with elevated HBV DNA levels and fluctuations in the serum ALT. Driven by mutations in the pre-core or core promoter regions of the virus (immune escape), HBeAg is not secreted and this disease phase is referred to as HBeAg-negative CHB.8 Similarly, patients may progress to HBeAg-negative CHB from the immune active disease phase following HBeAg seroconversion, if they fail to establish immune control of the virus exemplified by low levels of HBV DNA (<2,000 IU/ml) and normal liver enzymes (Fig 1 and 2).
Novel insights into disease phase
Current practice stratifies disease phase based exclusively on HBV DNA and ALT levels, however, there is a growing debate on the accuracy of these clinical parameters to reflect disease phase immunologically.9–11 The utility of qHBsAg, an indirect measure of transcriptional cccDNA, may enhance disease stratification (Fig 1). Cross-sectional studies demonstrate a variation in qHBsAg levels during the natural history of CHB and may enhance management decisions. Highest in immune tolerant disease, recent studies have shown an inverse correlation between qHBsAg level and liver fibrosis in HBeAg-positive disease.12 The lowest levels of qHBsAg are observed in HBeAg-negative ICs, where low qHBsAg levels reflect immune control and importantly, may help to risk stratify patients for disease progression and the development of HCC.13
Treatment and monitoring
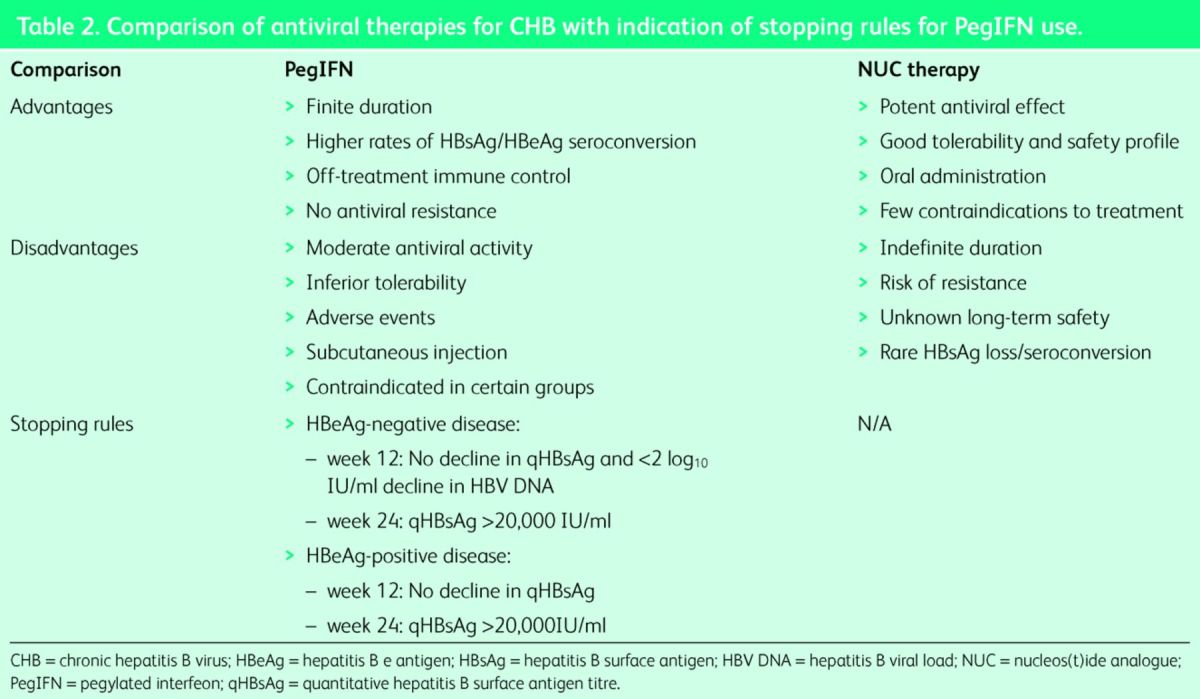
Through the inhibition of the HBV reverse transcriptase, NUCs (Table 2) provide on-treatment suppression of HBV replication. While offering potent viral suppression with a high genetic barrier to resistance, these drugs do not achieve cure owing to the persistence of cccDNA in infected hepatocytes. Conversely, PegIFN can result in virus clearance, but its efficacy is limited to a small proportion of patients and it can be associated with systemic side effects.5
Table 2.
Comparison of antiviral therapies for CHB with indication of stopping rules for PegIFN use.
The treatment sequence
NICE and international guidelines differ in their recommendations for first-line therapies. NICE recommends PegIFN as first-line treatment in adults with HBeAg-positive or negative compensated liver disease, with NUCs (tenofovir/entecavir) offered as a second line in those with a suboptimal response to PegIFN (Table 2). Both the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) offer more flexibility in treatment selection, with the choice of first-line agent being left to physician discretion.14,15 Prior to the initiation of any first-line therapy (PegIFN/NUC), specific laboratory tests should be performed to ensure there is no contraindication to the selected therapy and provide baseline measurements for on-treatment monitoring (Table 1).
Pegylated interferon
Advantages of PegIFN include a finite treatment course and the absence of antiviral resistance. Owing to its immunomodulatory activity, off-treatment immune control is achievable. Approximately 30% of HBeAg-positive patients have a favourable response to PegIFN with sustained HBeAg seroconversion, with a proportion of these patients going on to achieve HBsAg loss, the closest outcome to a clinical cure.16 Importantly, PegIFN also has a role in HBeAg-negative disease, where a sustained virologic response (HBV DNA <2,000 IU/ml) is seen in up to 40% of patients and HBsAg loss reported in approximately 12% at 5 years post treatment.17
Recently described early stopping rules (Table 2), based primarily on HBsAg decline at week 12 (or 24) of therapy, can guide physicians in determining where treatment can be stopped early owing to suboptimal response; thus avoiding the potential undesirable effects associated with a full-treatment course. This strategy would allow an early switch to NUC therapy, providing an individualised approach to CHB treatment.5,16,17
Nucleos(t)ide analogues
Tenofovir and entecavir represent third-generation NUCs and may be offered as a first-line therapy in CHB, or following suboptimal response to PegIFN.5 Associated with less systemic side effects, monitoring of patients on NUC therapy is mandated to assess response and detect potential adverse events associated with long-term therapy (Table 1). Recent studies have demonstrated histological improvement (reversal of fibrosis) with long-term NUCs.18 Importantly, there may also be a reduction in HCC development, but this needs to be substantiated in large clinical trials. Although NUCs are potent inhibitors of viral replication, they lack any significant impact on qHBsAg levels. Consequently, NUC therapy in the majority of patients in clinical practice is life long.19
Monitoring the untreated patient
Monitoring of non-treatment candidates is mandated to detect disease progression and determine the timing of any therapeutic intervention (Fig 2). The frequency of monitoring is debated, with a delicate balance between overzealous monitoring and the minimum frequency of follow up without putting patients at risk. The optimum monitoring of immune-tolerant patients remains unclear; while three-monthly laboratory tests are likely to capture fluctuations in biochemical activity or viral load, it may take many years for progression of disease from the tolerant to the immune-active phase. Recent work in ICs has demonstrated how qHBsAg can enhance monitoring and identify those at greatest risk of disease progression (reactivation) or the development of HCC. In this disease phase, low levels of qHBsAg define a ‘low-risk’ IC profile where monitoring frequency can be reduced.12,13 Similarly, integrating qHBsAg into disease profile to define immune tolerance could potentially streamline the frequency of monitoring in this group.
The cirrhotic patient
HBV-related decompensated cirrhosis is an absolute indication for NUC therapy, which can adequately suppress viral replication, potentially reverse fibrosis, prevent liver transplantation and even the development of HCC.18 Lifelong use of NUCs in cirrhotic patients is the standard of care to prevent complications of CHB. Conversely, PegIFN is an absolute contraindication in decompensated disease. In addition to the routine laboratory monitoring, similar to that in the non-cirrhotic patient, cirrhotic patients should undergo screening for HCC with six-monthly surveillance ultrasound scan (+/–) α-fetoprotein (AFP) (Table 1).
Novel treatment strategies in CHB
Novel strategies to maximise the efficacy of currently available treatments, including the combination of these agents or their use in sequence, are currently under investigation.20,21 Eradication of the virus is the ideal therapy goal. Novel agents with promise include viral entry inhibitors, targets against cccDNA formation and degradation. In addition, strategies to enhance the host immune response against HBV and the role of therapeutic vaccination are under investigation. The majority of these strategies are at a pre-clinical stage and their potential therapeutic impact remains unknown.22,23 Furthermore, these strategies are likely to be combined with NUCs and/or PegIFN in the short term, therefore the therapies used in today's clinic are likely to constitute a central component of treatment strategies for the foreseeable future.24
Summary
CHB remains a major global healthcare challenge, accounting for more than 50% of the cases of primary liver cancer worldwide. The complexity of CHB and its dynamic nature necessitate specialist input to make appropriate treatment and management decisions. However, current therapies remain suboptimal and fail to achieve HBsAg loss, the ‘gold-standard’ treatment endpoint, in the majority of patients. In light of the recent advances in HCV treatment, there is a need for similar progress in the HBV therapy field to make eradication of the virus a realistic prospect. In order to achieve this goal, novel agents must be developed in addition to optimising the use of currently available agents. The central tenet, however, remains a better understanding of disease phase and timing of therapeutic intervention.
References
- 1 .Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582–92. [DOI] [PubMed] [Google Scholar]
- 2 .National Institute for Health and Care Excellence NICE issues guideline on the diagnosis and management of chronic hepatitis B. London: NICE, 2013. [PubMed] [Google Scholar]
- 3 .Bertoletti A, Rivino L. Hepatitis B: future curative strategies. Curr Opin Infect Dis 2014;27:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 .Martinot-Peignoux M, Asselah T, Marcellin P. HBsAg quantification to optimize treatment monitoring in chronic hepatitis B patients. Liver Int 2015;35 Suppl 1:82–90. [DOI] [PubMed] [Google Scholar]
- 5 .Sonneveld MJ, Janssen HL. Chronic hepatitis B: peginterferon or nucleos(t)ide analogues? Liver Int 2011;31 Suppl 1:78–84. [DOI] [PubMed] [Google Scholar]
- 6 .Hui DK, Leung N, Yuen ST, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune tolerant phase. Hepatology 2007;46:395–401. [DOI] [PubMed] [Google Scholar]
- 7 .Liaw YF, Chu CM, Su IJ, et al. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology 1984;84:216–9. [PubMed] [Google Scholar]
- 8 .Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e-antigen negative chronic hepatitis B –natural history and treatment. Semin Liver Dis 2006;26:130–41. [DOI] [PubMed] [Google Scholar]
- 9 .Kennedy PT, Sandalova E, Jo J, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012;143:637–45. [DOI] [PubMed] [Google Scholar]
- 10 .Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol 2014, epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 .Gill US, Kennedy PT. Chronic hepatitis B virus in young adults: the need for new approaches to management. Expert Rev Anti Infect Ther 2014;12:1045–53. [DOI] [PubMed] [Google Scholar]
- 12 .Jaroszewicz J, Calle Serrano B, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 2010;52:514–22. [DOI] [PubMed] [Google Scholar]
- 13 .Tseng TC, Liu CJ, Yang HC, et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 2013;57:441–50. [DOI] [PubMed] [Google Scholar]
- 14 .European Association For The Study Of The Liver EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167–85. [DOI] [PubMed] [Google Scholar]
- 15 .Lok ASF, McMahon BJ. Chronic hepatitis B: update 2009 – AASLD practice guidelines. Hepatology 2009;50:1–36.19554618 [Google Scholar]
- 16 .Sonneveld MJ, Hansen BE, Piratvisuth T, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology 201;58:872–80. [DOI] [PubMed] [Google Scholar]
- 17 .Marcellin P, Bonino F, Yurdaydin C, et al. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int 2013;7:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18 .Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468–75. [DOI] [PubMed] [Google Scholar]
- 19 .Chevaliez S, Hezode C, Bahrami S, et al. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol 2013;58:676–83. [DOI] [PubMed] [Google Scholar]
- 20 .Gill US, Peppa D, Micco L, et al. Functional innate immune responses are restored with sequential NUC therapy following pegylated interferon–alpha exposure and not with NUC monotherapy in chronic hepatitis B. Hepatology 2014;60:1030A–1021A. [Google Scholar]
- 21 .Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014;61:777–84. [DOI] [PubMed] [Google Scholar]
- 22 .Zoulim F. Are novel combination therapies needed for chronic hepatitis B? Antiviral Res 2012;96:256–9. [DOI] [PubMed] [Google Scholar]
- 23 .Koh S, Bertoletti A. Circumventing failed antiviral immunity in chronic hepatitis B virus infection: triggering virus-specific or innate-like T cell response? Med Microbiol Immunol 2014, epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24 .Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol 2013;58:205–9. [DOI] [PubMed] [Google Scholar]