Abstract
Medicine has always striven to personalise or stratify approaches towards individual patients, but recently these terms have been applied particularly to denote improved disease sub-classification achieved through new genetic and genomic technologies. Techniques to analyse a person's genetic code have improved in sensitivity exponentially over recent years and at the same time the cost of such analyses has become affordable to routine NHS care. This article highlights the significant opportunities that genomics brings to healthcare, as well as some of the practical and ethical challenges.
Key Words: genomics, genetic testing, ethics, public health, health care, personalised medicine, stratified medicine, health professional
Introduction
The central role of the medical professional is to diagnose disease and provide advice on treatment. The physician does this as accurately as possible and works with the patient to ensure that the choice of management fits with personal preferences and circumstances, and takes into account issues such as comorbidities, as well as physiological and psychological characteristics. Skilled clinical history-taking and physical examination remain essential as the basis of this activity, aided by investigations such as radiological or biochemical tests. Technological advances over the past few decades mean that such investigations now can be refined, or even replaced in some cases, by the measurement of genetic or genomic biomarkers. The molecular characteristics of a disorder or the genetic make-up of an individual can fine tune a diagnosis and inform its management. These new capabilities, often termed ‘stratified’ or ‘personalised’ medicine, are likely to have a profound effect on the practice of medicine and service delivery.
Genetic medicine, which uses genetic or genomic biomarkers in this way, has, until recently, been the province of a small minority of specialised physicians who have used it to diagnose or assess risk of inherited disease. Recognition that most disease has a genetic component, the development and application of new genetic tests to identify important disease subsets and the availability of cost-effective interventions mean that genetic medicine must be integrated more widely across healthcare services. In order to optimise benefit equitably across the population, physicians and services need to be ready to change and adapt to new ways of working.
This paper summarises the expected impact of emerging genomic technologies on health services, as well as some of the key challenges these technologies pose.
The impact of genomic technologies on diagnosis and care
The increasing ease and speed of genome analysis, along with its rapidly decreasing cost, have increased knowledge of the genetic variations between individuals and their relationship with health and disease. Methods for DNA sequencing have seen massive transformations, and novel techniques will have far-reaching impacts on diagnostic abilities within the NHS. The recent House of Lords report on genomic medicine1 and the report of the Human Genome Strategy Group (HGSG)2 were predicated on the assumption that whole-genome sequencing would soon become relatively commonplace within medical practice and would transform investigations for a diversity of conditions – from infectious disease control to susceptibility testing for common diseases.
Next-generation sequencing technologies also enable diagnosis of single-gene disorders to be made more readily, particularly when responsible genes are large or the conditions are genetically heterogeneous (that is, alterations may be in any one of a number of different genes). Currently, genes are often tested sequentially, so results can take many months and the cost may be prohibitive to NHS services. Techniques that allow several genes to be tested at the same time at significantly reduced costs will therefore have a major impact on diagnostic services. The tally on the international Genetests website by August 2012 shows that it is now possible to test for almost 2,700 single-gene disorders.3 These tests have increasing utility, being linked to particular prognostic advice, preventive or treatment options, testing of relatives and prenatal testing to determine whether or not a condition has been transmitted to offspring.
Although single-gene disorders are individually rare, they are collectively common, affecting more than 3.5 million people (one in 17 of the population) in the UK;4 they often exist as subgroups of more common conditions and occur in most body systems. The child with developmental delay and the patient with diabetes, high cholesterol, a heart arrhythmia or deteriorating vision may each have an inherited condition. They must be diagnosed and managed by a physician who understands the condition, the different treatment approaches for subsets of the disease, the impact for relatives who may also be at risk (and the importance of identifying affected relatives) and the best forms of holistic management. The chronic and often severe nature of such conditions means that they also have a disproportionate representation in terms of attendances at emergency departments and hospital admissions.5 Table 1 provides examples of common presentations of diseases with a strong underlying genetic component, with examples of why molecular diagnosis is important. In all cases, the relevance to family members will be a significant factor. A molecular diagnosis made in a young child may allow parents to consider prenatal or preconception options to avoid having a second affected child.
Table 1.
Common conditions and genetically predisposed subgroups.
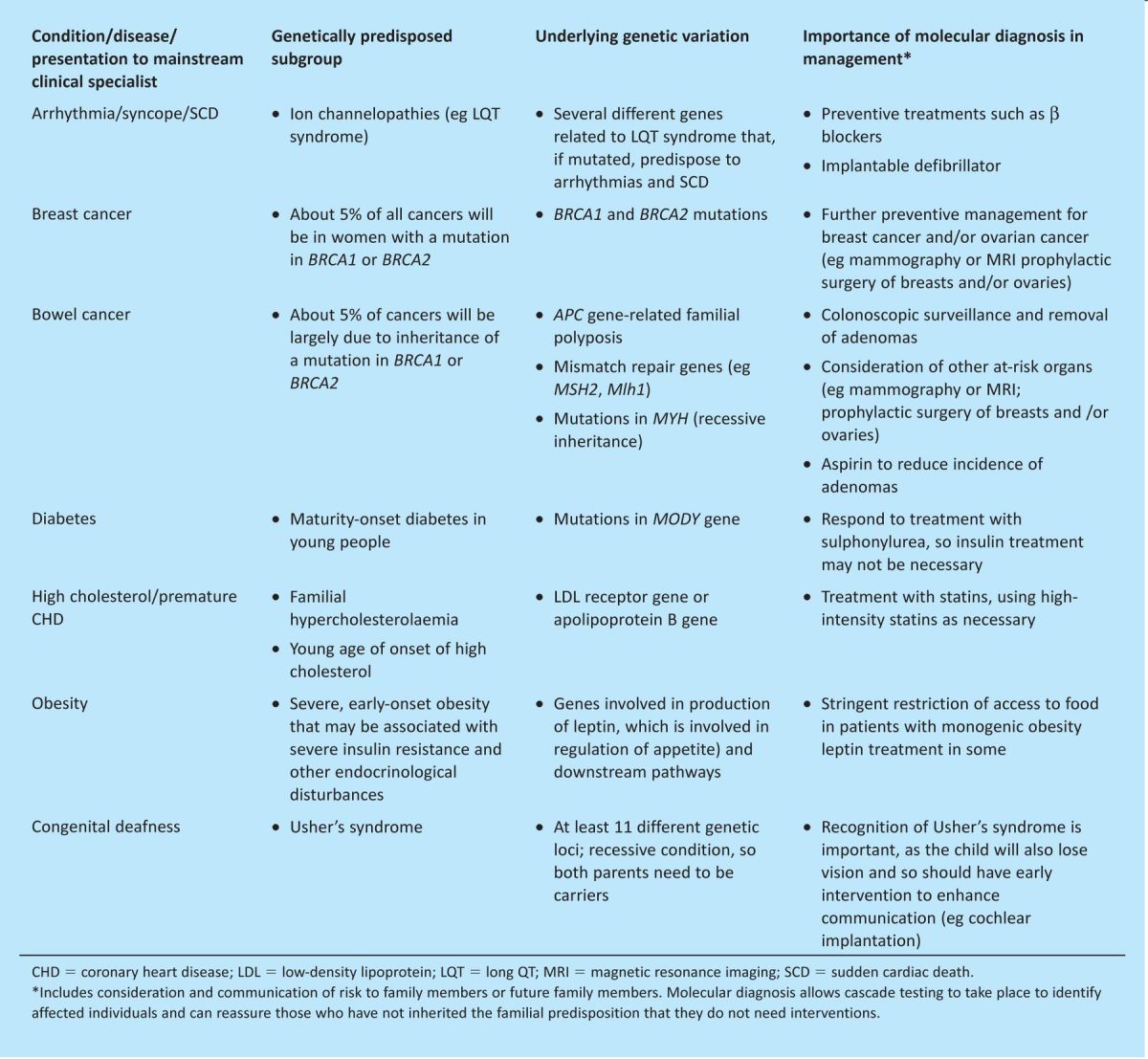
As well as delineating inherited components, genomic technologies can identify the genetic characteristics of acquired diseases, such as cancer, and thereby inform prognosis, treatment and surveillance. Molecular characterisation of tumours can be helpful in individualisation of cancer management, resulting in stratified treatment of cancer.
Elucidation of the relatively rare single gene subsets of complex disorders will have implications for different treatments, but the characterisation of genomic biomarkers in multifactorial conditions is also becoming more helpful in the stratification of populations to provide preventive options for certain subgroups. For example, the Collaborative Oncological Gene-environment Study (COGS), which is funded by the European Commission FP7, is exploring the effectiveness of integrating genetic and environmental data to improve risk prediction and to develop stratified prevention strategies for breast, prostate and ovarian cancer.6 The successful implementation of stratified prevention programmes will require the involvement of a wide range of physicians – from primary to specialist care.
The challenges
Effective implementation of genomics will test the healthcare system as a whole. The HGSG report2 and a recent report on whole-genome sequencing by the Foundation for Genomics and Population Health (PHG Foundation),7 an independent policy research organisation, outlined many of the challenges. These include the development of the necessary scientific evidence base that will support clinical interpretation of genomewide sequence data. This will require standardised databases of normal and pathogenic genomic variations linked to analytical tools that will facilitate clinical decision making and the development of the necessary clinical bioinformatics expertise and infrastructure to enable clinical interrogation of genomic sequence data. This is no small task. Although research has been incredibly successful at identifying areas of the genome that may increase or decrease susceptibility to common conditions by, for example, a few percentage points, the interaction of these risk factors, whether they act multiplicatively or additively or are dependent on particular environmental or stochastic factors, still requires much research before clear clinical utility can be attributed.
Challenges to the NHS include: the development of the appropriate laboratory configuration and capabilities; good practice guidance to ensure clear understanding between doctor and patient of what will be tested, what will not be tested and why; and the development of approaches that protect confidentiality while harnessing the ability of genetics to predict risks for relatives as well as index patients. Clinicians will need to know when and how results from one person should be communicated to their relatives, how complex, potentially familial information can best be conveyed to ensure valid consent and how enduring such consent should be, given that multiple investigations can be applied to a small sample that is stable over many years. Furthermore, clinicians must remain sensitive to the fact that although some patients wish to know about their genetic inheritance, others do not, and some may choose not to have genetic testing at all.
Perhaps the greatest challenge is to ensure the readiness of physicians to use these genomic technologies for maximum effect, so that genetic medicine is incorporated into mainstream specialties. For some clinicians, particularly those involved in clinical research, these advances are already a reality. However, a sizable majority do not yet recognise the relevance of genetics for their clinical practice, perceiving genetic conditions to be rare and untreatable. Maximising genomic opportunities also means being aware of their limitations: media portrayals that indicate that genetic information gives clear-cut answers are often unrealistic. Indeed, knowing one's entire genomic sequence is not the crystal ball of our future that many hope it to be, and physicians will need to be more familiar with what is hype and what is reality for the integration of genetics into mainstream medicine to be successful.
In June 2011, clinicians from a wide range of specialties met at the Royal College of Physicians in London to discuss the challenges for diagnosing and managing inherited disorders as they present in mainstream medical practice. The meeting was organised and led jointly by the Joint Committee on Medical Genetics (JCMG), UK Genetic Testing Network (UKGTN), National Genetics Education and Development Centre (NGEDC) and PHG Foundation. Clinicians from 15 specialties were represented, together with clinical and laboratory geneticists, primary care physicians, commissioners and representatives of patient groups. The meeting particularly addressed the question of how excellent practice, developed in some centres and based on close working between genetics services and particular specialties, could be generalised across the full range of clinical specialties and the country as a whole. A report of this meeting was published in June 2012.8 Delegates at the meeting agreed that major advances in understanding of inherited disorders within various areas of clinical medicine have been achieved through very close collaboration of clinicians and researchers with a special interest in inherited conditions. Throughout the country, such research has led to service developments, often in the form of ‘joint’ services that integrate particular clinical areas with clinical genetic expertise. Work in inherited cardiac conditions, for example, showed that the specialist knowledge of the cardiologist in interpreting complex arrhythmias has to be complemented by the expertise of the geneticist in diagnosing inherited syndromes, interpreting genetic tests and dealing with the implications for family members.
Evidence from reviews of specialised services in inherited cardiovascular disease and ophthalmology has shown that the comprehensive services developed in a small number of centres are not replicated consistently throughout the UK and that major disparities exist in the volume and nature of provision.9 Bridging the resulting service gap will be a major challenge for the professions and will require attention to professional education; evidence-based commissioning; ethical, legal and social elements; communication; and patient engagement.
Education for clinicians must meet the needs of generalists, as well as those who will provide more specialised advice and care. Those with a subspecialist interest will be an essential component of the multidisciplinary team looking after patients with inherited disease and must have an appropriate level of specialised knowledge, experience and ongoing clinical exposure. The subspecialty committees of the royal colleges with responsibility for education and training should take a professional lead in determining educational need and developing required resources and opportunities within their specialty training programmes.
The development and commissioning of new care pathways for inherited disease should take place as an integral part of provision within major disease programmes – for example, those concerned with cardiovascular disease, cancer, diabetes, renal disease, etc. Identification of those who should instigate such testing, and should therefore carry the clinical and financial responsibility, will vary in different clinical settings, but it should be based on testing criteria such as those produced by the UKGTN.10
As the range of genomic tests increases, the cost decreases and the clinical utility improves, genetic testing will be undertaken by a widening group of disease specialists, who must understand how to use and interpret such tests to gain clinical utility for their patient. The physician should seek specialist advice on genetics as appropriate – for example, for recognition of syndromes and for detailed assessment of patterns of inheritance or complexities in the use and interpretation of genetic tests. An important consideration in the commissioning of genetic services is that measurement of their value is complex, involving more than their cost and resultant clinical interventions: for example, there may be clinical utility in providing information and contextualising anxieties about family history, and long-term outcomes of surveillance measures are difficult to measure, particularly for relatively rare conditions, as is the impact of cascade (family-tracing) screening. A complex investigation in one person may result in accurate predictive tests in several relatives, thus targeting interventions more efficiently. One person may use genetic testing to guide their medical management, but a relative may use it to influence reproductive decision making and avoid the birth of an affected child; these complexities not only require skilled professional interactions, but their cost effectiveness is difficult to measure.
For diseases with a strongly inherited component, the management of family members who may also be at risk poses significant challenges to 21st-century medical practice, which tends to treat individual patients and places a high value on individual consent and confidentiality. The question of what duties or obligations, if any, are owed to relatives, and by whom, will exercise physicians as genetics is integrated into mainstream clinical practice.11
The development of new commissioning guidance related to inherited disorders also comes at a time of maximum upheaval in NHS commissioning structures, with processes in place to transfer responsibility to a large number of clinical commissioning groups and the NHS Commissioning Board (NHSCB) directly commissioning specialised services, including those for clinical genetics. It will be vital that commissioning arrangements recognise the role of the specialty of clinical genetics in providing support to various other clinical specialties. It may be more difficult to ensure that the pathways for the main population-level programmes adequately incorporate the need to recognise and effectively manage important disease subsets, including inherited diseases. Clinical networks (set to be a feature of the new commissioning arrangements) must be formed to ensure that appropriate professional advice is given and should include mainstream clinicians with a special interest in inherited disease, as well as genetic specialists.
Finally, both professionals and the public should have a realistic view of what is possible. Although the discovery of genetic risk factors in common diseases such as heart disease and cancer has led to important insights about disease mechanisms, the predictive power of individual genetic variants is often very low. Developments in bioinformatics will need to evolve considerably before the identification of a particular combination of genetic variants in an individual will have clinical utility for them.
Conclusion
Diagnosis and management of inherited disorders provides one very tangible example of stratified medicine – the tailoring of intervention to a particular molecular understanding of the disease in an individual and their family. There is little doubt that the landscape of diagnosis and management will change rapidly over the next 10–15 years, with genetic and other biomarkers increasingly used to aid decision making. Health services will see a rapid evolution of pathology requirements, which must be paralleled by development of individuals with skills to use these tests and interpret them intelligently for the good of patients and their families. The royal colleges should be at the forefront of these changes, advocating for the most effective service provision and leading and ensuring the highest level of professional development.
References
- 1.House of Lords Science and Technology Committee. Genomic medicine, volume 1: report. London: Stationery Office, 2009 [Google Scholar]
- 2.Human Genomics Strategy Group. Building on our inheritance: genomic technology in healthcare. London: DH, 2012 [Google Scholar]
- 3.GeneTests. www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests [Accessed 1 August 2012]
- 4.Donaldson L. Rare is common. Annual report of the Chief Medical Officer. London: DH, 201039–45 [Google Scholar]
- 5.Kumar P, Radhakrishnan J, Chowdhary MA, Giampietro PF. Prevalence and patterns of presentation of genetic disorders in a pediatric emergency department. Mayo Clin Proc 2001;76:777–83 [DOI] [PubMed] [Google Scholar]
- 6.Pashayan N, Duffy SW, Chowdhury S, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer 2011;104:1656–63 10.1038/bjc.2011.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright C, Burton H, Hall A, et al. Next steps in the sequence: the implications of whole genome sequencing for health in the UK. Cambridge: PHG Foundation, 2011. www.phgfoundation.org/pages/wholegenome.htm [Accessed 1 August 2012] [Google Scholar]
- 8.Burton H, Cole T, Farndon P.Genomics in Medicine: Delivering genomics through clinical practice. London: PHG Foundation, 2012. Report on the Joint Committee on Medical Genetics. [Google Scholar]
- 9.Burton H, Alberg C, Moore AT. Genetics in ophthalmology: equity in service provision. J Public Health (Oxf) 2010;32:259–66 10.1093/pubmed/fdp110 [DOI] [PubMed] [Google Scholar]
- 10. NHS UK Genetic Testing Network, Testing Criteria, 2009. www.ukgtn.nhs.uk/gtn/Information/Services/Testing_Criteria [Accessed 29 August 2012]
- 11.Royal College of Physicians. Consent and confidentiality in clinical genetic practice: guidance on genetic testing and sharing genetic information, second edition, London: RCP, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


