Key Points
The prevalence of atopic dermatitis has trippled over the last 30 years and cost the UK economy £465 million per annum
Loss of function mutations in the fillagrin gene are strongly associated with severe atopic dermatitis phenotypes
Systemic treatment by a dermatologist should be considered when optimised topical treatment has failed to control the disease
Ciclosporin, azathioprine and methotrexate all have RCT evidence supporting their use in severe atopic dermatitis
Emerging treatments in atopic dermatitis include monoclonal antibodies against IgE and IL5
Atopic eczema and atopic dermatitis (AD) are synonymous terms for atopic dermatitis, a chronic inflammatory skin condition characterised by a red itchy rash. It commonly affects the skin flexures leading to a thickening of the skin with increased skin lines known as lichenification. There is excoriation with associated crusting, scaling and weeping of the skin. The AD seen in adult populations most commonly presents in childhood and persists. However, more rarely it can present de novo in adult life. It is estimated that the prevalence of AD has at least tripled over the last 30 years, costing the UK economy £465 million a year in health costs and lost working days.1 Its negative impact on quality of life includes poor sleep, time off work and social ostracism. The AD phenotype is a separate clinical and pathogenic entity from other eczema subtypes such as seborrhoeic, irritant, contact, venous or discoid eczema. The diagnostic criteria are detailed in Table 1.2
Table 1.
The UK refinement of Hanifin and Rajka's diagnostic criteria for atopic dermatitis.2

It has long been recognised that atopic dermatitis runs in families and confirmed by the discovery of several candidate genes associated with atopic disease. Identifying these loci led to the discovery of the filaggrin gene on chromosome 1q21 which encodes for an important structural protein in maintaining the skin's normal barrier function.3 Loss-of-function mutations in this gene are strongly associated with the more severe phenotype of AD and its persistence into adulthood.4 It is estimated that 60% of childhood atopic dermatitis resolve by adulthood.1 Other factors that predict severe adult AD include onset at an early age with severe and widespread disease in infancy as well as concurrent atopic disease such as asthma and seasonal rhinitis.
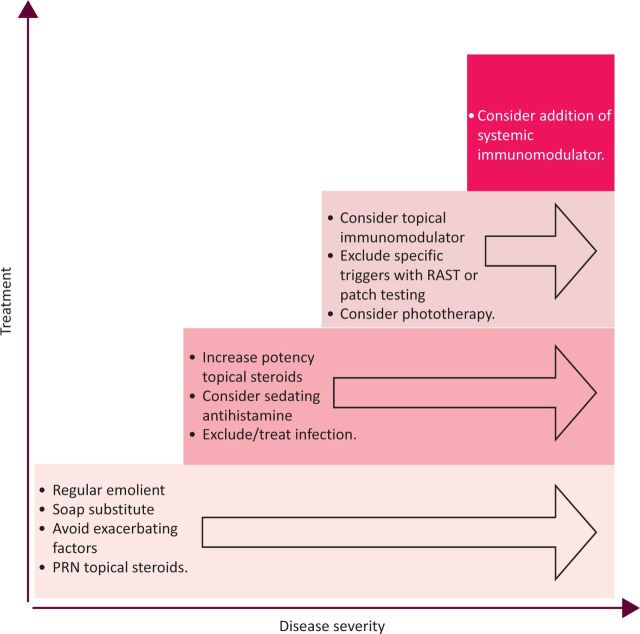
Figure 1 illustrates the pathogenesis of AD. This article reviews the treatment options available for those adult patients who are unable to control their disease despite optimal topical management with skin directed therapies. This cohort of patients should always be under the management of a dermatologist. Reasons for failure of skin directed therapies should be considered before considering systemic treatment. These include ensuring treatment concordance, excluding recurrent or antibiotic-resistant infection and consideration of a more intensive topical treatment regimen. Although less common in adult disease, specific allergic triggers or exacerbants should also be excluded from the history, and relevant diagnostic tests considered (eg radio-allergosorbent test (RAST) or patch testing).
Fig 1.
Stepwise approach to the management of atopic dermatitis (PRN = as required; RAST = radio-allergosorbent test).
Phototherapy, including narrow band ultraviolet B (UVB) and psoralen UVA range (PUVA), has been shown to be effective in AD5,6 and is an alternative option to systemic therapy. Consideration should be given to the additional potential risk of skin cancer in patients receiving phototherapy who are likely to progress to treatment with systemic immunosuppressants.
Established systemic treatments
A stepwise approach to the management of AD is shown in Fig 1.
Antihistamines
Although the pruritus associated with AD is not solely a histamine-driven process, antihistamines can be useful in treating any urticaria or dermographism associated with a specific allergic response. Long established H1-receptor antagonists can also prove useful in controlling nocturnal itch through their sedating properties.
Oral corticosteroids
The use of a short course of oral prednisolone to control a severe exacerbation is extremely effective and remains commonly used in clinical practice. Long-term oral corticosteroids given to control disease are inevitably complicated by predictable toxicity and have been superseded by newer immunomodulators with better side effect profiles. In a recent randomised controlled trial (RCT) of oral prednisolone versus ciclosporin in severe AD, the latter was more efficacious in maintaining a stable remission.7
Ciclosporin
Ciclosporin is a well established systemic treatment in AD and one of the only therapies licensed for this use. It is a fungus-derived immunosuppressant initially developed for use in transplant recipients. Its mode of action is potent inhibition of T-lymphocyte-driven immune responses and production of interleukin (IL)-2. Clinical trials have consistently shown ciclosporin to be a highly efficacious treatment in AD, with an average 50% reduction in disease severity and significant improvement in quality of life.8 It is well tolerated and its rapid onset of action makes it a popular first-line choice in the treatment of refractory AD.
Adverse effects
There is however a significant risk of hypertension and nephrotoxicity with ciclosporin. Most patients experience a rise in baseline creatinine of approximately 20%.9 Appropriate patient selection and careful monitoring of creatinine, biochemistry and blood pressure are therefore mandatory throughout treatment. There is also the well documented increased risk of non-melanoma skin cancers (NMSC) in long-term use, further exaggerated in patients who have previously undergone PUVA. It is these risks that often preclude the use of ciclosporin as a long-term maintenance therapy. Most commonly ciclosporin is prescribed as a 6–9 month treatment course. Unfortunately, the rates of relapse are high on stopping ciclosporin, an estimated 50% relapsing by two weeks and 80% at two months.10
Azathioprine
Azathioprine (AZA) is a further example of an immunosuppressant drug initially developed for use in transplant recipients. The evidence for AZA as an effective treatment of AD is building and two double-blind RCTs have confirmed a modest treatment benefit with, on average, a 37% improvement in disease severity markers.11,12
AZA is metabolised by three main pathways (Fig 3). Identifying patients with reduced thiopurine methyltransferase (TPMT) activity allows for appropriate dose reduction to decrease the risk of fatal myelosuppression. It is thought that reduced or absent TPMT activity accounts for 10% of the cases of myelosuppression associated with AZA. Therefore TPMT activity should be checked prior to commencing any patient on AZA, but this does not replace the need for regular haematological monitoring.13
Fig 2.
Pathogenesis of atopic dermatitis. The healthy stratum corneum allows the retention of fluid and provides an immune barrier to aeroallergens and infection. Defects in the structural binding protein filaggrin disrupt the barrier function, leading to loss of moisture in the stratum corneum, increased surface pH, loss of resistance to staphylococcus and increased allergen priming. Dendritic cells act as antigen presenters to naive T-cells which then recruit further T-cell populations (predominantly Th2) through the release of pro-inflammatory cytokines. Allergen sensitisation then results in the presence of specific immunoglobulin E antibodies which bind antigen and cross link on the surface of mast cells causing degranulation and histamine release (the ‘atopic response’).
Fig 3.
Summary of the major metabolic pathways of azathioprine (AZA). HGPRT = hypoxanthine guanine phosphoribosyl transferase; MeMP = methylmercaptopurine; MP = mercaptopurine; TGN = thioguanine nucleotide; TIMP = tissue inhibitor of metalloproteinases; TPMT = thiopurine methyltransferase; TU = thiouric acid; XO = xanthine oxidase; MeMpN = methylmercaptopurine nucleotide).
Unlike ciclosporin, azathioprine takes several weeks to reach a steady state in the blood and is therefore slow to take effect. Once patients are established on treatment, it is generally well tolerated and can be continued for longer courses than ciclosporin.
Adverse effects
TPMT status does not predict the frequency of other major adverse effects associated with AZA therapy, including nausea, hypersensitvity and hepatotoxicity. As with ciclopsorin, an increased risk of NMSC has been observed in transplant recipients on long-term treatment. It is proposed that this is via AZA-induced increased photosensitivity, so photoprotection should be advised in all patients on treatment. Whether AZA also leads to an increased risk of lymphoma remains controversial. This has been observed in transplant recipients, but it remains to be seen whether it is a side effect of the immunosuppression per se rather than of the drug.
Methotrexate
Methotrexate is a well established treatment in psoriasis but also useful in AD. It is not an immunosuppressant at the low doses used in inflammatory disorders but is postulated to interfere with T-cell responses. This led to the hypothesis that it should also be beneficial in AD, another T-cell-driven disease. Two open case series have shown promising results using low-dose methotrexate as a treatment for moderate to severe AD. A retrospective study reported a response in 75% of patients after receiving methotrexate for three months,14 and a prospective trial confirmed a significant treatment benefit (>50% improvement) for most patients after a similar duration.15 A recently published RCT compared 12 weeks' treatment with methotrexate or azathioprine in 42 patients.16 Both showed clinically relevant therapeutic benefit with similar response rates, thereby indicating methotrexate as an alternative first-line systemic treatment in severe AD.
Adverse effects
Most patients tolerate methotrexate well. Chances of bone marrow suppression, hepatotoxicity and gastrointestinal (GI) side effects can be reduced with folic acid supplementation.
Mycophenolate mofetil and mycophenolate sodium
A potent lymphocyte selective immunosuppressant is available in two different forms: mycophenolate mofetil (MMF) and, more recently, mycophenolate sodium (MPS) available in an enteric-coated form (EC-MPS). This latter formulation is designed to decrease the GI side effects caused by the mycophenoloic acid itself.
Original open-label studies of MMF in AD reported promising results with most patients having a significant response after 4–8 weeks' treatment.17,18 However these results have not always been reproducible. More recently, an unblinded RCT was published comparing EC-MPS with ciclosporin as a maintenance treatment for moderate to severe AD.19 There was similar efficacy in both groups but the onset of treatment benefit was delayed in those on EC-MPS. On completing the treatment course, rates of relapse were higher in the ciclosporin group, suggesting EC-MPS may have a more prolonged treatment effect. The side effect profile of MMF also compared favourably to that in the ciclosporin group, with no cutaneous infections reported.
Emerging systemic treatments
Biologic therapies
Biologic therapies comprise protein-based molecules produced by living organisms that either inhibit or mimic naturally occurring human proteins. The older biologic therapies include interferon (IFN) and intravenous immunoglobulin (ivIg). The evidence for ivIg as a treatment is limited,20 and its high cost means that it is now rarely used. IFN shows a modest treatment benefit,20 but the ‘flu-like’ side effects and cost also preclude its widespread use.
Monoclonal antibody biologics
It is the newer field of monoclonal antibodies (mABs) that offers the most exciting new treatment avenues. Several of the newer mAB biologics have been trialled in AD and there are a number of case reports of the anti-tumour necrosis factor agents adalimumab, etanercept and infliximab in AD.21 However, inconsistent findings and the understanding that AD is a predominantly (T helper) Th2- rather than Th1-driven response has meant that no larger scale studies have been conducted.
A newer generation of mABs towards IgE and eosinophilic pathways has gathered more interest, not least because of the dramatic treatment responses seen in the other atopic diseases, allergic asthma and allergic rhinitis.
Omalizumab
Omalizumab is a humanised monoclonal mouse antibody against IgE. It has shown promising results in the treatment of IgE-driven diseases such as allergic asthma and allergic rhinitis. These results have proved difficult to replicate consistently in AD. There are several case reports of significant clinical improvements with omalizumab in AD associated with a high total IgE.22 However, in a recent double-blind RCT in 20 patients omalizumab did not significantly alter the clinical disease severity parameters despite decreasing free serum IgE.23
Mepolizumab
Similarly, early small-scale trials of mepolizumab, a new humanised mAB to IL-5 (an important cytokine in the production and maturation of eosinophils) have shown only modest benefit in reducing the severity of AD despite a marked reduction in the circulating eosinophil count.24
The lacklustre improvements in clinical outcomes for AD with omalizumab and mepolizumab, both of which are proven to decrease IgE activity, suggest that the pathogenesis of chronic AD is more complex than that of a purely IgE driven ‘atopic’ response. If these emerging treatments are to have a role in AD, it may be in treating acute forms of the disease or when a specific IgE hypersensitivity has been identified.
Alitretinoin
Two large-scale RCTs with alitretinoin, a new vitamin A derivative belonging to the retinoid group of drugs, have shown a substantial treatment benefit in chronic hand eczema patients. It is now a licensed treatment for this indication. It was postulated therefore that it may also be of benefit in extrapalmar AD. To date, large-scale randomised trials have been recommended in the literature, but have not yet commenced. Preliminary findings from a open-label study of six patients found a substantial clinical improvement in the extrapalmar manifestations of AD over a 12-week period, with a promising side effect profile.25 The current levels of evidence for systemic treatments in atopic dermatitis are summarised in Table 2.
Table 2.
Levels of evidence for systemic treatments for atopic dermatitis.

Summary and future outlook
AD in its severe form has a huge impact on patients' quality of life. Its prevalence in the western world continues to increase and therapeutic options at present are aimed only at control not cure. Most of the current systemic treatments available effect only a modest improvement and therefore should be used in addition to the already established ongoing topical treatments.
As more is understood about the pathogenesis of AD, it may become possible to identify at a young age those individuals likely to progress to lifelong severe disease. By instituting rigorous supportive care to maintain the skin barrier it may be possible to prevent hypersensitisation and its chronic disease sequelae. In the meantime, the search continues for a drug that is highly efficacious but non-toxic, allowing an effective and safe long-term treatment option for those who suffer with this disabling chronic disease.
References
- 1.Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Review. Health Technol Assess 2000;4:1–191 [PMC free article] [PubMed] [Google Scholar]
- 2.Williams HC, Burney PG, Pembroke AC, Hay RJ. Validation of the U.K. diagnostic criteria for atopic dermatitis in a population setting. U.K. Diagnostic Criteria for Atopic Dermatitis Working Party. Br J Dermatol 1996;135:12–7 [PubMed] [Google Scholar]
- 3.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441–6 10.1038/ng1767 [DOI] [PubMed] [Google Scholar]
- 4.Barker JN, Palmer CN, Zhao Y, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol 2007;127:564–7 10.1038/sj.jid.5700587 [DOI] [PubMed] [Google Scholar]
- 5.Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol 2000;25:552–8 10.1046/j.1365-2230.2000.00700.x [DOI] [PubMed] [Google Scholar]
- 6.Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001;357:2012–6 10.1016/S0140-6736(00)05114-X [DOI] [PubMed] [Google Scholar]
- 7.Schmitt J, Schäkel K, Fölster-Holst R, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol 2010;162:661–8 10.1111/j.1365-2133.2009.09561.x [DOI] [PubMed] [Google Scholar]
- 8.Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema – a systematic review and meta-analysis. Review. J Eur Acad Dermatol Venereol 2007;21:606–19 10.1111/j.1468-3083.2006.02023.x [DOI] [PubMed] [Google Scholar]
- 9.Berth-Jones J, Graham-Brown RA, Marks R, et al. Long-term efficacy and safety of cyclosporin in severe adult atopic dermatitis. Br J Dermatol 1997;136:76–81 [PubMed] [Google Scholar]
- 10.Schmitt J, Schäkel K, Schmitt N, Meurer M. Systemic treatment of severe atopic eczema: a systematic review. Acta Derm Venereol 2007;87:100–11 10.2340/00015555-0207 [DOI] [PubMed] [Google Scholar]
- 11.Berth-Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol 2002;147:324–30 10.1046/j.1365-2133.2002.04989.x [DOI] [PubMed] [Google Scholar]
- 12.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet 2006;367:839–46 10.1016/S0140-6736(06)68340-2 [DOI] [PubMed] [Google Scholar]
- 13.Meggitt SJ, Anstey AV, Mohd Mustapa MF, Reynolds NJ, Wakelin S. British Association of Dermatologists' guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol 2011;165:711–34 10.1111/j.1365-2133.2011.10575.x [DOI] [PubMed] [Google Scholar]
- 14.Goujon C, Bérard F, Dahel K, et al. Methotrexate for the treatment of adult atopic dermatitis. Eur J Dermatol 2006;16:155–8 [PubMed] [Google Scholar]
- 15.Weatherhead SC, Wahie S, Reynolds NJ, Meggitt SJ. An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol 2007;156:346–51 10.1111/j.1365-2133.2006.07686.x [DOI] [PubMed] [Google Scholar]
- 16.Schram ME, Roekevisch E, Leeflang MM, et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol 2011;128:353–9 10.1016/j.jaci.2011.03.024 [DOI] [PubMed] [Google Scholar]
- 17.Murray ML, Cohen JB. Mycophenolate mofetil therapy for moderate to severe atopic dermatitis. Clin Exp Dermatol 2007;32:23–7 10.1111/j.1365-2230.2006.02290.x [DOI] [PubMed] [Google Scholar]
- 18.Neuber K, Schwartz I, Itschert G, Dieck AT. Treatment of atopic eczema with oral mycophenolate mofetil. Br J Dermatol 2000;143:385–91 10.1046/j.1365-2133.2000.03667.x [DOI] [PubMed] [Google Scholar]
- 19.Haeck IM, Knol MJ, Ten Berge O, et al. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol 2011;64:1074–84 10.1016/j.jaad.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 20.Smith DI, Swamy PM, Heffernan MP. Off-label uses of biologics in dermatology: interferon and intravenous immunoglobulin (part 1 of 2). Review. J Am Acad Dermatol 2007;56:e1–54 [DOI] [PubMed] [Google Scholar]
- 21.Graves JE, Nunley K, Heffernan MP. Off-label uses of biologics in dermatology: rituximab, omalizumab, infliximab, etanercept, adalimumab, efalizumab, and alefacept (part 2 of 2). J Am Acad Dermatol 2007;56:e55–79 10.1016/j.jaad.2006.07.019 [DOI] [PubMed] [Google Scholar]
- 22.Lane JE, Cheyney JM, Lane TN, et al. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol 2006;54:68–72 10.1016/j.jaad.2005.09.030 [DOI] [PubMed] [Google Scholar]
- 23.Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges 2010;8:990–8 10.1111/j.1610-0387.2010.07497.x [DOI] [PubMed] [Google Scholar]
- 24.Oldhoff JM, Darsow U, Werfel T, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 2005;60:693–6 10.1111/j.1398-9995.2005.00791.x [DOI] [PubMed] [Google Scholar]
- 25.Grahovac M, Molin S, Prinz JC, et al. Treatment of atopic eczema with oral alitretinoin. Br J Dermatol 2010;162:217–8 10.1111/j.1365-2133.2009.09522.x [DOI] [PubMed] [Google Scholar]
Bibliography
- 1. Breathnach SM, Smith CH, Chalmers RJ, Hay RJ. Burns T, Breathnach SM, Cox N, Griffiths C. Systemic therapy. Rook's Textbook of Dermatology 2010. 8th edn Oxford: Wiley-Blackwell [Google Scholar]
- 2.Friedmann PS, Ardern-Jones M, Holden CA. Burns T, Breathnach SM, Cox N, Griffiths C. Atopic dermatitis. Rook's Textbook of Dermatology 2010. 8th edn Oxford: Wiley-Blackwell [Google Scholar]