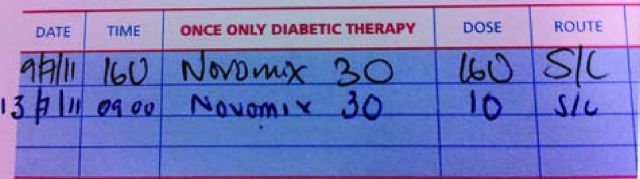ABSTRACT
Insulin therapy is important in many patients with diabetes, but the UK National Diabetes Inpatient Audit (NaDIA) suggests that insulin therapy in hospital is poorly monitored and managed. Although most hospitals should have access to an inpatient diabetes specialist team, it is important for the non-specialist clinician to be aware of the indications for insulin therapy, types of insulin and insulin regimens, methods of adjusting insulin doses and the need for care with insulin prescribing. Here, we demystify issues around insulin therapy.
KEYWORDS: Diabetes, insulin therapy
Introduction
Diabetes is common, and becoming more so. At the present rate of increase, the current 3 million people with diabetes will increase to over 5 million in the UK by 2025.1 Approximately one in five inpatients in UK hospitals have diabetes, and these patients have higher mortality and morbidity, and longer length of stay compared with non-diabetic individuals.2
All doctors will manage patients with diabetes within their caseload. However, the annual National Diabetes Inpatient Audit (NaDIA) showed that management of diabetes among inpatients is poor.2 In particular, adjustment of insulin therapy according to blood glucose levels is poorly managed. Many junior doctors lack confidence with insulin commencement or adjustment.3 Therefore, here, we demystify insulin therapy for the non-diabetes physician.
When is insulin therapy necessary?
Type 1 diabetes mellitus and ketoacidosis
Insulin is necessary for life in patients with type 1 diabetes mellitus (T1DM). Presentation with typical acute symptoms (polyuria, polydipsia and weight loss) without diabetic ketoacidosis (DKA) should prompt same-day insulin initiation by a specialist team, which can usually be managed as an outpatient. DKA requires emergency admission and management.
DKA is not only seen in patients with new-onset or existing T1DM, but is also recognised in patients with type 2 diabetes mellitus (T2DM), especially in people of African and Caribbean descent (ie with ketosis-prone diabetes),4 and people taking atypical antipsychotics.5 It is salutary to find that, in the most recent NaDIA survey, 63 patients (0.4%) had developed DKA after their admission to hospital.2
Recent British guidelines give detailed strategies for managing DKA.6 They also state that, in DKA in existing T1DM, the basal long-acting insulin should be continued alongside intravenous insulin and mealtime bolus reintroduced on recovery, which aids rapid transition to subcutaneous insulin, although not all diabetes units agree with this protocol.
Type 2 DM
Poor glycaemic control in T2DM is defined by the National Institute for Health and Care Excellence (NICE) as ‘when control of blood glucose remains or becomes inadequate (glycated haemoglobin (HbA1c) ≥7.5% or 58 mmol/mol or other higher level agreed with the individual) with other measures’.7 NICE recommends insulin as a third-line agent ‘for a person on dual therapy who is markedly hyperglycaemic… unless there is strong justification not to.’ Such justification for delaying insulin and trying a different third-line agent would include particular concern to avoid weight gain and jeopardising a patient's occupation, such as driving large vehicles.
Inpatients and ‘stress hyperglycaemia’
Inpatient stays are prolonged in those with diabetes.8 Although poor glycaemic control is associated with poorer outcomes, addressing this during hospitalisation with intensive insulin has yielded disappointing benefits in situations other than infections.9 Nevertheless, insulin might be required to address the deterioration in glycaemic control caused by sepsis, enteral feeding, steroid treatment or withdrawal of metformin (see below).
Insulin is useful as an effective temporary measure to achieve rapid control of ‘stress hyperglycaemia’, a hormonally driven catabolic stress of illness in people with no previous history of diabetes and a normal HbA1c. Similarly, temporary insulin use might be indicated in hyperglycaemia associated with parenteral nutrition and in steroid-induced diabetes.10
Surgery
Poor glycaemic control before elective surgery increases the risk of morbidity (especially infection), and length of stay.11 Patients with HbA1c above 70 mmol/mol will usually be encouraged to improve control before elective surgery takes place. This will often involve insulin, temporarily if not permanently. Careful perioperative planning can avoid intravenous insulin altogether if no more than one meal is missed, but if the surgery requires a starvation period across two or more meals, a variable-rate intravenous insulin infusion is needed. Emergency surgery will usually require perioperative intravenous insulin.
Other specific circumstances
Other specific indications for insulin therapy include:
infections, such as infected diabetic foot ulcers
acute coronary syndrome (ACS): intensive insulin is no longer recommended for all patients with hyperglycaemia when admitted with ACS,12 but insulin infusions are generally used to control hyperglycaemia exceeding 11 mmol/l during the first 48 h post event. Controversy remains around the duration of insulin treatment subsequent to this
critical care: overly tight glycaemic control has been associated with adverse outcomes in the critical care setting.13 Intravenous insulin with a target blood glucose of <10 mmol/l is generally accepted as a safe management strategy in the critical care setting
pregnancy: NICE has approved the use of metformin and glibenclamide in gestational diabetes,14 but insulin remains the mainstay of treatment for both gestational and pre-existing diabetes during pregnancy
cystic fibrosis: insulin is generally favoured in cystic fibrosis-related diabetes because it helps prevent weight loss.15
What are the alternatives to insulin therapy?
Sulfonylureas
A sulfonylurea can be a useful, rapid-acting alternative to insulin if a patient with T2DM is not already taking one. In preparation for surgery, a sulfonylurea can improve HbA1c in patients where its long-term use was being avoided to minimise weight gain. However, the incidence of hypoglycaemia is significant in inpatients taking sulfonylureas,16 and acute kidney injury (AKI) will further increase this risk. In the event of hypoglycaemia, sulfonylurea washout is prolonged.
Metformin
Metformin is the treatment of choice for T2DM in the outpatient setting but has less of a role in inpatient care.Gastrointestinal adverse effects limit the speed of dose escalation and, hence, its usefulness in the acute situation. Metformin use often needs to be suspended in patients with AKI (or chronic kidney disease with an estimated glomerular filtration rate (eGFR) <30 ml/min), acute liver injury (eg alcoholic hepatitis), or following iodinated contrast administration. Metformin reduces cardiovascular risk in patients with T2DM17 and, hence, its reintroduction into a patient's therapy should be planned once renal function is stable above a eGFR of 30 ml/min, and the acute illness is resolving. In the event of AKI, it should be explained to the patient that metformin was withdrawn because of its risks in kidney disease not because it caused the renal failure (which is a common misconception).
Glucagon-like peptide-1 agonists and dipeptidyl peptidase-4 inhibitors
Glucagon-like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP-4), two categories of drugs acting via the incretin (gut hormone-mediated) response, are attractive because of their low risk of hypoglycaemia. The DPP-4 inhibitors (‘gliptins’) are administered orally, and GLP-1 agonists (exenatide, liraglutide and lixisenatide) are injected. Although they rarely cause hypoglycaemia, and can induce significant weight loss, they are still undergoing trials of cardiovascular safety; for example, saxagliptin has been linked in this context to an excess of admissions for cardiac failure.18 Their introduction is better undertaken outside hospital rather than in the acutely ill. It is also important to dispel any misunderstanding that injected GLP-1 agonists are in some way insulin substitutes.
Sodium–glucose co-transporter-2 inhibitors
Sodium–glucose co-transporter-2 (SGLT-2) inhibitors (dapagliflozin and canagliflozin) work primarily by inhibiting glucose reabsorption in the proximal renal tubules, reducing the blood glucose threshold for glycosuria and thereby expelling increased amounts of excess glucose. They lower blood glucose with weight loss and minimal risk of hypoglycaemia, but again are associated with uncertain cardiovascular safety. They are avoided in patients with eGFR <50 ml/min, and have adverse effects including urinary tract infections and genital candidiasis.
Types of insulin
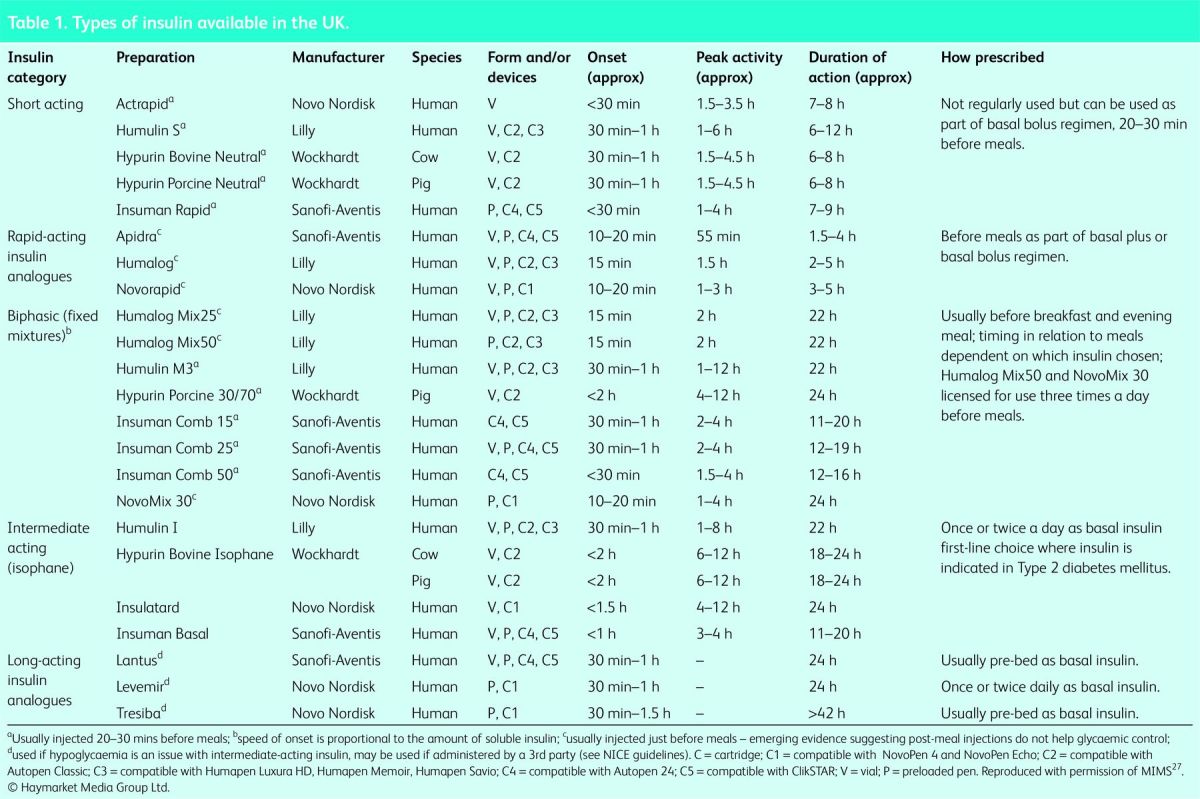
The predominant insulins in use in the UK are either synthetic analogues or human insulins. Insulins of animal origin (porcine or bovine) are still available, but are now used in only a few patients. There are five major types of insulin, according to pharmacokinetic profile (Table 1):
Table 1.
Types of insulin available in the UK.
rapid acting
short acting
intermediate acting (also called isophanes)
fixed mixtures (of rapid- or short-acting plus intermediate acting in various ratios)
long acting.
The current insulins available in most UK formularies are listed in Table 1. They can be prescribed in a confusing array of prefilled and reusable pens. The choice of device depends on the patient's capabilities and ease of teaching. However, cartridges and pen needles are not compatible with all reusable insulin delivery devices. Queries around pen devices should always be discussed with the diabetes specialist team.
Prescribing insulin
Many drug-related errors both in and outside of hospital are related to insulin prescription errors. When prescribing insulin, physicians should take care to prescribe the correct insulin name (commonly NovoMix 30 and Novorapid are confused as are Humalog, Humalog Mix 25 and Humalog Mix 50). Care should also be taken with timing (pre meal, pre bed, etc). A further common error is the use of ‘U’, ‘IU’ or ‘units’ added to the number. ‘U’ can be confused for ‘0’ and it has been reported that a patient received 10 times their prescribed insulin dose as a result of this confusion (Fig 1).
Fig 1.
A confusing insulin prescription. On 9 July 2011, this patient received a dose of 160 units of insulin and developed severe hypoglycaemia as a result. The second prescription on 13 July 2011 is correctly written.
Adverse effects of insulin
Hypoglycaemia
The major risk of insulin is iatrogenic hypoglycaemia. Patient education is central to its avoidance alongside careful choice of insulin regimen, including insulin analogues and insulin pumps. Inpatient hypoglycaemia is common, affecting 20% of inpatients in England and Wales in 2013.2 Alarming as this might sound, it represents a significant improvement since 2011. Further reduction will require high standards of blood-glucose monitoring, realistic targets for blood glucose of 6–10 mmol/l (acceptable range 4–12 mmol/l) and staff education.11 Insulin has been described as a ‘high-risk’ medication and errors are twice as likely to cause harm as any other prescribed treatment.19
Local and allergic reactions
True insulin allergy is rare but local reactions to additives are somewhat more common.20 The usual approach is to try a different brand of insulin, but occasionally skin-prick testing is required.
Weight gain
In outpatient clinics and primary care, weight gain as a consequence of the anabolic nature of insulin is a significant problem. Overweight patients generally prefer to avoid insulin and professionals work hard to avoid insulin in such patients because of spiralling weight and insulin requirements. In acute illness, with its attendant catabolic state, this is not usually a concern, but when planning discharge it is important to clarify to patients and their primary care team whether the insulin is intended for short- or long-term use.
Standard insulin regimens
There are several ways in which insulin can be prescribed, depending on the clinical circumstance and patient preference. Where insulin is started in patients with T2DM, there is good evidence that continuation of metformin is useful because it can limit weight gain and reduce the required dose of insulin.21 Metformin is also the one drug that has been shown to reduce cardiovascular mortality and morbidity in patients with T2DM.17
Basal insulin plus tablets
A common way to start insulin in patients with uncontrolled T2DM is the use of once-daily basal insulin (usually given at night before bed) in combination with oral medication, which is continued unchanged. Physiologically, this reduces nocturnal hepatic gluconeogenesis (the ‘leaky liver’) and suppresses fasting hyperglycaemia. Thus, for example, if a patient is uncontrolled on maximum tolerated sulfonylurea, metformin and gliptin, the addition of once-daily basal insulin would be a simple way to start insulin. This has the advantage of being relatively easy to administer, teach and titrate. A typical starting regimen would be an intermediate-acting insulin (Table 1) started once daily before bed. There is no strong evidence that analogue insulins are superior to human insulins in this regimen, and they are significantly more expensive.
A typical starting dose might be 10 units or related to body weight, but this is rapidly titrated every 2–3 days according to fasting glucose levels. In one large randomised controlled study, once-daily insulin regimens led to slightly poorer glucose control compared with other regimens, but with less hypoglycaemia and weight gain.22
Twice-daily fixed mixtures
A twice-daily fixed mixture regimen is generally reserved for patients with T2DM. It might be useful in patients with significant hyperglycaemia (particularly post-prandial hyperglycaemia) uncontrolled on tablets, or in patients uncontrolled on once-daily insulin. Insulin is administered before breakfast and the evening meal. Typically, a mixed insulin is used (mixed short and intermediate acting). The number on the insulin refers to the proportion of short-acting insulin present (eg NovoMix 30 or Humulin M3 = 30%, Humalog mix 25 = 25%).
In this regimen, sulfonylureas and gliptins would generally be stopped, but metformin would be continued. A standard starting dose would be 12 units in the morning and 8 units in the evening, although more might be given in patients who are requiring high doses of intravenous insulin, or are overweight or obese.
Titration of twice-daily insulin regimens should be undertaken on the basis of regular tests of fasting glucoses and pre-evening meal glucoses. If fasting glucoses are above target (usually 4–8 mmol/l), then the evening glucose dose should be increased by 5–10%. If the pre-evening meal glucoses are high, the morning insulin dose should be titrated.
Twice-daily fixed mixtures have the advantage of managing fasting and post-prandial hyperglycaemia, and are simple to teach, administer and titrate. Two injections as opposed to four injections daily might be attractive to some patients. The disadvantage of this regimen is that it lacks flexibility to enable changes in diet or exercise regimens. Carbohydrate intake should be approximately the same and at the same time each day. Snacking before bed might be required if nocturnal hypoglycaemia is an issue. In some circumstances, biphasic insulin can be used three times a day.
Basal bolus regimen
The most physiological regimen for insulin administration is the use of basal insulin, usually given at night, or sometimes split between night and morning, along with boluses of rapid-acting insulin given before meals. This regimen is used in most patients with T1DM, and in some patients with T2DM who are inadequately controlled on other regimens. In T2DM, a ‘basal plus’ regimen can be used, whereby rapid-acting insulin is added at one or two meals to help with post-prandial glucose control. The basal insulin is generally given as a long-acting analogue or intermediate-acting human insulin, and the bolus is more conveniently given as rapid-acting analogue.
With the exception of metformin, other oral hypoglycaemics are generally stopped when converting to a basal bolus regimen. In a patient newly found to have T1DM and naïve to insulin, starting regimens can be undertaken by weight, or by the amount of intravenous insulin infused over the preceding 24–72 h (see below).
Continuous subcutaneous insulin infusion
Continuous subcutaneous insulin infusion (CSII or insulin pumps) are used in patients with T1DM, and only initiated in specialist centres where there is a multidisciplinary team to support the patient. NICE guidance states that continuous subcutaneous insulin infusion is recommended as an option for patients with T1DM provided that multiple-dose insulin therapy has failed to achieve good glycaemic control, and those receiving the treatment have the commitment and competence to use the therapy effectively.23 The pumps are particularly useful in patients with poor hypoglycaemia awareness. Insulin pumps are delivery devices, rather than artificial pancreases. Their programming and adjustment is done by the user of the pump and their healthcare professionals. Pump failure can rapidly lead to DKA, and any concern about an inpatient's fitness to deal with their pump should prompt the substitution of intravenous insulin and referral to a specialist team.
Variable rate insulin infusions and intermittent (‘as required’) insulin
Previously called ‘sliding scales’, variable rate insulin infusions (VRII) are commonly used in inpatients with diabetes, sometimes inappropriately. The aim of a VRII is to maintain glucose levels between 6–10 mmol/l for most of the time, although 4–12 mmol/l is acceptable.24 VRII are used in diabetic patients taking insulin who are undergoing elective procedures, or are admitted acutely, and oral intake of nutrient is erratic or uncertain. There is no one size fits all, and some patients who are insulin resistant might need rapid titrations in dose. The Joint British Societies guidelines on managing patients with diabetes undergoing elective procedures give clear guidance on how and when to use VRII.24 As a rule, a VRII should be run concurrently with appropriate intravenous fluids, and be curtailed as soon as possible, once the patient is eating and drinking.
Insulin given ‘as required’ in patients with diabetes is not good practice. Although a junior doctor might be faced with the situation of a diabetic patient with acute hyperglycaemia, and a start dose of short-acting insulin might be required, frequent dosing of such insulin should not be sustained. It is important that such patients have appropriate adjustments of their pre-existing diabetes therapy, and this might require the involvement of the diabetes specialist team.
Initiating insulin
Starting insulin should be done by an expert, and it is important to involve the diabetes specialist team. Structured advice around insulin injection sites and/or technique, titration, timing, hypoglycaemia, driving, work, insurance, food, storage of insulin and disposal of sharps will need to be covered. There are several competency-based courses available to support clinicians to develop in this area of practice. The local diabetes specialist team will advise which course is commissioned in their area and the support available for clinical staff. The Royal College of Nursing has also published information to support clinicians initiating injectable therapies.25
With regard to initial dose, a variety of confusing algorithms for starting insulin in diabetic patients has been described. None are very evidence based and, hence, some simple rules of thumb can be applied. When starting basal or twice-daily insulin, it is reasonable to start low and rapidly titrate. Thus, 10 units of basal insulin, or 12 + 8 units of twice-daily mixed insulin are reasonable starting doses for most patients, as long as doses are titrated according to glucose monitoring. A diabetes nurse specialist will often teach the patient about self-titration of insulin doses.
In the case of patients with newly diagnosed T1DM converting to a basal bolus regimen, the total daily dose of insulin in such patients can be calculated from the amount infused intravenously over the previous 24 h if they have been eating and drinking normally, or based on their body weight. The total daily insulin dose in most patients is approximately 0.5 units/kg, of which most patients require approximately 40–60% as basal, and the rest as boluses split between meals. The basal insulin is increased according to fasting blood glucose levels, and mealtime bolus insulin according to preprandial glucose levels. Most patients with T1DM are taught carbohydrate counting to adjust their insulin dose according to their carbohydrate intake and prevailing glucose level.26 If a patient with established T1DM is admitted, it is usually safe to allow them to self-adjust insulin levels according to meals, and many hospitals offer this flexibility.
Adjusting insulin doses
Case 1 – optimisation presurgery
Mr MA, a 62-year-old with a 10-year history of T2DM had hip replacement surgery postponed because his HbA1c was 96 mmol/mol. At a preoperative diabetes service, his diet was reviewed and he was taught how to administer a 30/70 mixed insulin, starting with 12 units before breakfast and 8 units before his evening meal. The dose was increased in increments of 2 units twice a week until his target blood glucose profiles were obtained. After 3 months, his dose had reached 26 units am, 20 units pm, his HbA1c had improved to 65 mmol/mol and his orthopaedic surgery went ahead. Good glycaemic control is linked to improved postoperative outcomes and reduced complications.
Case 2 – short course of steroids
Ms RW, a 21-year-old with T1DM treated with a basal bolus insulin regimen, also had severe asthma requiring a short course of prednisolone 40 mg reducing to zero after 5 days. She increased her rapid-acting insulin doses at mealtimes by 20% per day until she finished the steroid course, but kept her basal dose unchanged. Her blood glucose rose above 15 most evenings. Whenever it was above 12, she checked her capillary ketones, which remained normal (<0.6).
Glucocorticoids predominantly affect post-prandial rather than fasting blood glucose levels. Thus, mealtime boluses will need to be increased as a steroid effect is seen and reduced on steroid withdrawal (although some impact remains for at least 24 h after withdrawal). In contrast, long-acting basal insulin might need more modest or no adjustment.
Case 3 – diabetic ketoacidosis
Ms LS was admitted with DKA probably precipitated by a viral illness. During resuscitation with intravenous fluids, intravenous insulin was given at a fixed rate of 8 units/h (based on an estimated weight of 80 kg and calculated as 0.1 unit/kg/h), but was increased to 9 units/h when her blood glucose had dropped less than the recommended 3 mmol/l/h after 3 h. Her usual basal insulin was continued at night throughout and her mealtime rapid-acting insulin was reintroduced before her lunch when she felt ready to eat again. The intravenous insulin was stopped 30 min later.
Case 4a – infection
Mr KC was admitted with cellulitis and a plantar neuropathic foot ulcer. His T2DM had been treated with gliclazide and metformin before admission with a most recent HbA1c of 70 mmol/mol. Instead of a variable-rate intravenous insulin infusion (‘sliding scale’), the inpatient diabetes team instituted a basal bolus regimen using rapid-acting insulin 8 units three times daily before meals and intermediate-acting basal insulin 16 units at night. After 3 days, his blood glucose were 7–8 mmol/l fasting, 3–5 mmol/l before lunch, 9–14 mmol/l before dinner and 7–11 mmol/l at 22.00 h. Priority was given to avoiding hypoglycaemia, recognising that the higher readings before his evening meal might have resulted from treatment of hypoglycaemia before lunch. Thus, his breakfast rapid insulin was reduced to 6 units (ie by 20%). Two days later, he was eating better and his blood glucose measured 9–12 mmol/l fasting, 8–13 mmol/l before lunch, 8–14 mmol/l before dinner and 9–16 mmol/l at 22.00 h. All four doses were increased by 10% (7 units before breakfast, 9 units before lunch and before his evening meal) and 18 units of intermediate insulin at night. His blood glucose stabilised within the target range of 6–10 mmol/l.
Case 4b – using mixed insulin
When Mr KC's discharge was planned, it had become evident that he would struggle to self-inject. His wife agreed to take responsibility but just twice daily because she had a lunchtime job at the local school. His doses outlined above yielded a total daily dose (TDD) of 43 units. By splitting his TDD, a regimen of twice-daily 30/70 mixed insulin was prescribed, 22 units pre-breakfast and 20 units pre-evening meal. The next day, his blood glucose dropped mid-afternoon, prompting a 20% reduction in his morning dose. He was discharged on 30/70 mixed insulin, 18 units pre-breakfast and 20 units pre-evening meal.
Conclusions
Insulin is an important therapy in patients with diabetes. It can not only be lifesaving in acute emergencies, but also a therapy that can enhance quality of life in patients with poorly controlled diabetes. Insulin therapy can be confusing to the uninitiated because of simple unfamiliarity. We hope that this article has illustrated that insulin is a safe, effective therapy, but that it must be prescribed carefully, and doses should be adjusted according to prevailing glucose levels.
References
- 1.Diabetes UK Diabetes in the UK in 2010: Key Statistics on Diabetes. London: Diabetes UK, 2010. [Google Scholar]
- 2.National Diabetes Inpatient Audit , 2013. www.hscic.gov.uk/catalogue/PUB13662/nati-diab-inp-audi-13-nat-rep.pdf [Accessed 30 September 2014]. [Google Scholar]
- 3.George JT, Warriner D, McGrane DJ, et al. Lack of confidence among trainee doctors in the management of diabetes: the Trainees Own Perception of Delivery of Care (TOPDOC) Diabetes Study. QJM 2011;104:761–6. 10.1093/qjmed/hcr046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra S, Oliver NS, Dornhorst A. Diabetic ketoacidosis: not always due to type 1 diabetes. BMJ 2013;346:f3501. 10.1136/bmj.f3501 [DOI] [PubMed] [Google Scholar]
- 5.Lean MEJ, Pajonk FG. Patients on atypical antipsychotic drugs: another high-risk group for type 2 diabetes. Diabetes Care 2003;26:1597–605. 10.2337/diacare.26.5.1597 [DOI] [PubMed] [Google Scholar]
- 6.Savage MW, Dhatariya KK, Kilvert A, et al. Diabetes UK Position Statements and Care Recommendations: Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diab Med 2011;28:508–15. 10.1111/j.1464-5491.2011.03246.x [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence Type 2 diabetes: the management of type 2 diabetes. NICE Guidelines CG87. London: NICE, 2009. [Google Scholar]
- 8.Diabetes Health Intelligence , 2011. Variation in inpatient activity: diabetes. Key findings for England. www.yhpho.org.uk/resource/item.aspx?RID=111515 [Accessed 30 September 2014]. [Google Scholar]
- 9.Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:49–58. 10.1210/jc.2011-2100 [DOI] [PubMed] [Google Scholar]
- 10.Jakoby MG1, Nannapaneni N. An insulin protocol for management of hyperglycemia in patients receiving parenteral nutrition is superior to ad hoc management. J Parenter Enteral Nutr 2012;36:183–8. 10.1177/0148607111415628 [DOI] [PubMed] [Google Scholar]
- 11.NHS Diabetes, 2011. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. www.diabetes.org.uk/Documents/Professionals/Reports%20and%20statistics/Management%20of%20adults%20with%20diabetes%20undergoing%20surgery%20and%20elective%20procedures%20-%20improving%20standards.pdf [Accessed 30 September 2014]. [Google Scholar]
- 12.National Institute for Health and Care Excellence Hyperglycaemia in acute coronary syndromes: Management of hyperglycaemia in acute coronary syndromes. NICE Guidelines CG130. London: NICE, 2011. [PubMed] [Google Scholar]
- 13.The NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–97. 10.1056/NEJMoa0810625 [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence Diabetes in -pregnancy: management of diabetes and its complications from pre-conception to the postnatal period. NICE Guidelines CG63. London: NICE, 2008. [Google Scholar]
- 15.Onady GM1, Stolfi A. Insulin and oral agents for managing cystic fibrosis-related diabetes. Cochrane Database Syst Rev 2013;7:CD004730. [DOI] [PubMed] [Google Scholar]
- 16.Rajendran R, Kerry C, Rayman G, on behalf of the MaGIC study group Temporal patterns of hypoglycaemia and burden of sulfonylurea-related hypoglycaemia in UK hospitals: a retrospective multicentre audit of hospitalised patients with diabetes. BMJ Open 2014;4:e005165. 10.1136/bmjopen-2014-005165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10 year follow up of intensive glucose control in Type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 18.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 19.Lamont T, Cousins D, Hillson R, Bischler A, Terblanche M. Safer administration of insulin: summary of a safety report from the National Patient Safety Agency. BMJ 2010;341:c5269. 10.1136/bmj.c5269 [DOI] [PubMed] [Google Scholar]
- 20.Heinzerling L, Raile K, Rochlitz H, Zuberbier T, Worm M. Insulin allergy: clinical manifestations and management strategies. Allergy 2008;63:148–55. 10.1111/j.1398-9995.2007.01567.x [DOI] [PubMed] [Google Scholar]
- 21.Hemmingsen B, Christensen LL, Wettersley J, et al. Comparison of metformin and insulin versus insulin alone for type 2 diabetes: Systematic review of randomised clinical trials with meta-analyses and trial sequential analyses. BMJ 2012;344:e1771. 10.1136/bmj.e1771 [DOI] [PubMed] [Google Scholar]
- 22.Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes for the 4-T study group. N Eng J Med 2007;357:1716–30. 10.1056/NEJMoa075392 [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus. NICE technology appraisal TA151 London: NICE, 2008. [Google Scholar]
- 24.Diabetes UK, 2011. Management of adults with diabetes undergoing elective procedures: improving standards. www.diabetes.org.uk/About_us/What-we-say/Improving-diabetes-healthcare/Management-of-adults-with-diabetes-undergoing-surgery-and-elective-procedures-improving-standards/ [Accessed 30 September 2014]. [Google Scholar]
- 25.Royal College of Nursing Starting injectable treatment in adults with type 2 diabetes: RCN guidance for nurses. London: RCN, 2012. [Google Scholar]
- 26.Keen AJ, Duncan E, McKillop-Smith A, Evans ND, Gold AE. Dose adjustment for normal eating (DAFNE) in routine clinical practice: who benefits? Diabet Med 2012;29:670–6. 10.1111/j.1464-5491.2011.03479.x [DOI] [PubMed] [Google Scholar]
- 27.MIMS Insulin Preparations. Available online at www.mims.co.uk/news/1096962/Insulin-Preparations [Accessed 7 October 2014]. [Google Scholar]