Abstract
In chronic obstructive pulmonary disease (COPD) a pathophysiological cycle occurs such that locomotor muscle weakness and fatiguabilty exist, which in turn limit exercise performance both because of leg discomfort and also because anaerobic metabolism leads to lactic acid production. Since the lactic acid is buffered by bicarbonate there is consequent carbon dioxide (CO2) production. Patients with advanced COPD are flow limited and cannot excrete the CO2 by raising ventilation and thus these patients experience breathlessness which discourages exercise and, in turn, prompts further deconditioning. Structured exercise, termed pulmonary rehabilitation is at the core of reversing the cycle but novel strategies should be employed for patients with advanced disease and alternative therapeutic opportunities may soon be available to improve pulmonary mechanics.
Introduction
Chronic obstructive pulmonary disease (COPD) remains the most common pulmonary disease of adults. COPD is responsible for over 25,000 deaths in the UK annually and is predicted by 2020 to be the third leading cause of death and the fifth leading cause of disability worldwide.2,3 The mainstays of current drug treatment are long-acting bronchodilators, both antimuscarinic and b-agonists and, in many patients, inhaled corticosteroids, but regrettably many patients remain symptomatic despite using these therapies. Moreover, there is no evidence that these treatments either prolong survival or reduce the rate of lung function decline. Thus novel approaches are urgently required.
Previously, a conceptual model was proposed where quadriceps dysfunction leads, as a result of anaerobic metabolism, to increased ventilatory requirements and thus dyspnea; such a cycle would be self-reinforcing since it would tend to lead to reduced physical activity and greater deconditioning.1 In this article, aspects of the problem within the framework of an earlier lecture by Michael Polkey will be discussed,1 concentrating specifically on new data.
Locus of exercise limitation
It was previously noted that cycling tasks were associated with leg discomfort in a much larger proportion of patients with COPD than walking tasks, but that in either case low frequency fatigue of the quadriceps was present in some patients.4 Since that time additional data have become available which strengthen the concept by seeking to remove one locus. Thus, in an elegant study Pepin and colleagues were able to show that a nebulised bronchodilator increased walking time but not cycle time because, being an airway drug, it did not act on the locus of exercise limitation.5 Few COPD patients in the UK cycle, although more do in continental Europe (10% of GOLD IV patients in the Copenhagen City Heart Study), the relevance of determining the locus of exercise limitation is that weight bearing activities, such as stair climbing or rising from a chair, do depend on quadriceps function. The locus of exercise limitation is now being actively studied in a number of initiatives worldwide including the PROactive project (www.proactivecopd.com) which has two UK centres.
Quadriceps myopathy
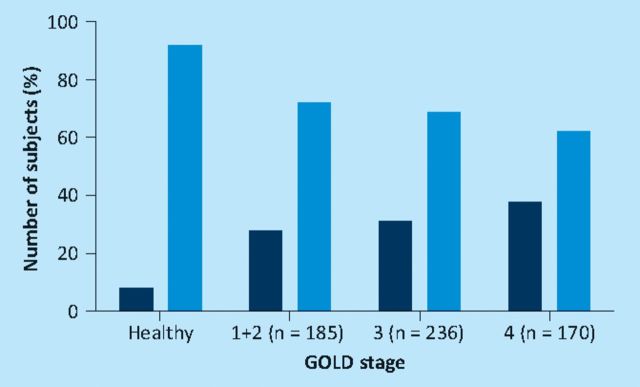
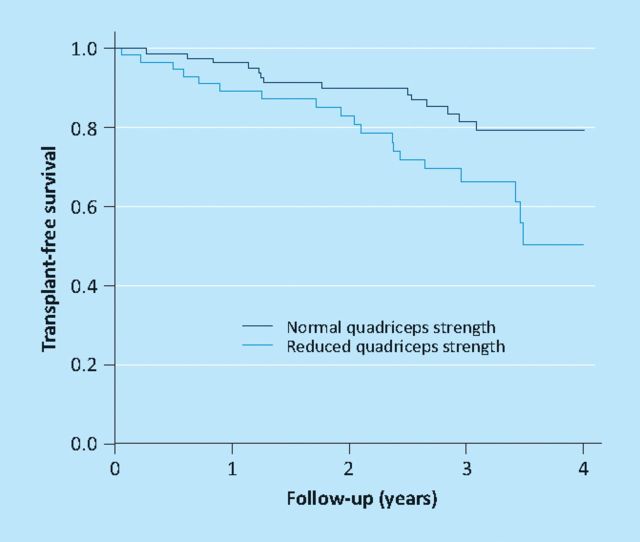
Quadriceps myopathy in COPD was previously considered an epiphenomena of advanced disease, and in that situation to be common. However, this assumption relied in part on the fact that normal ranges for quadriceps strength were poorly defined. To address this problem, data sets from control subjects studied at the Royal Brompton and Kings College Hospitals over a 10-year period were combined. Based on this data set of 212 participants in an age range suitable for COPD studies, regression equations were presented allowing quadriceps strength to be calculated as a percentage of a predicted value; factors needed for correction were age, height, fat-free mass and gender.6 These values were then applied to 591 COPD patients drawn from the London sites and from collaborators in Maastricht. Unexpectedly it was found that quadriceps weakness was quite common across all GOLD stages (COPD is classified into four stages of severity by the volume of air expired in the first second of a forced expiratory manoeuvre (FEV1) so that I is the least severe and IV the most) rising non-significantly from 31% in GOLD I/II to 38% in GOLD IV (Fig 1). Since physical activity is increasingly recognised as an aetiological factor in quadriceps weakness7 these observations are also consistent with the notion that physical activity measured objectively using a triaxial accelerometer is also variable and reduced in some patients with early COPD.8 It has also been established that among patients with GOLD III/IV, quadriceps strength with age is a better predictor of mortality than FEV1 (Fig 2); indeed below 50% predicted FEV1 does not seem to wield an effect on survival.9 The immobility concept is strengthened by data showing similar changes to those reported in other non-COPD pulmonary conditions, such as severe scoliosis.10 Conversely, since 2005, the likelihood that systemic inflammation contributes to quadriceps weakness has been downplayed since evidence of muscle inflammation is sparse11 and, at best, confined to a subset of patients.12
Fig 1.
Prevalence of quadriceps weakness in chronic obstructive pulmonary disease according to GOLD stage. Dark bars = participants with quadriceps less than the lower limit of normal; light bars = participants with quadriceps strength in the normal range. Adapted with permission of the European Respiratory Society ©.6
Fig 2.
Transplant free survival as a function quadriceps strength. Data from reference 9.
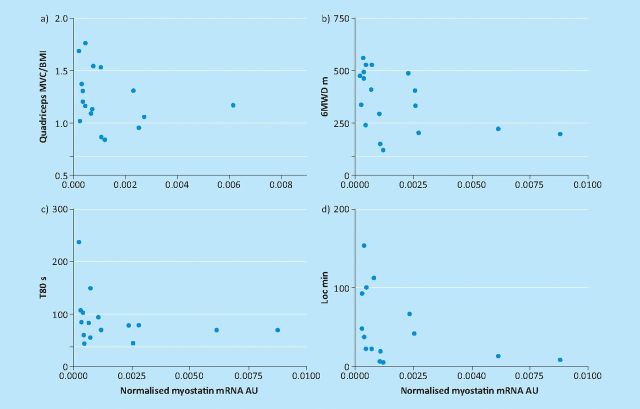
Since 2005 some progress has also been made in unravelling the pathophysiologic processes linked to atrophy/hypertrophy signalling in the skeletal muscle of patients with COPD. In animal models, many classically used pathways (eg immobility, sepsis, burns) culminate in over expression of ubiquitin ligases, especially MURF and atrogin, which ‘tag’ muscle fibres for destruction. Thus there was excitement in 2007 when Doucet and colleagues reported over-expression of ubiquitin ligases in patients with COPD,13 suggesting that the same mechanisms could be active. Subsequently it has become clear that this concept is over simplistic, particularly with the demonstration that ubiquitin ligase expression paradoxically increases rather than decreases after rehabilitation in cachectic COPD patients.14 Myostatin signalling seems to be a more robust candidate pathway; myostatin is a negative regulator of muscle mass so that in the farming world mutations which result in reduced myostatin expression are valued because they produce ‘double-muscled’ breeds including the Piedemont cow and the Texel sheep. In COPD myostatin is reduced following pulmonary rehabilitation14; conversely in this cohort levels were found to be highest in those patients who were weakest, least physically active and had the shortest six-minute walking distance (Fig 3).15
Fig 3.
Quadriceps myostatin RNA expression in chronic obstructive pulmonary disease patients as a function of (a) strength, (b) six-minute walk, (c) endurance time and (d) physical activity measured using triaxial accelerometry. Reproduced with permission of the European Respiratory Society ©.15
Status of current treatment opportunities in the spiral
Preventing myopathy
It was previously reported that training given soon after acute exacerbation could reduce readmission rate.16 Because of uncertainties about the mechanism of action, as well as changes in the UK healthcare system, the study was repeated in an entirely different and slightly larger cohort. On this occasion17 superiority of the intervention over usual care was again shown so that the chance of being readmitted with a further exacerbation of COPD was 33% in the usual care arm and 7% in the early pulmonary rehabilitation group (p=0.02). Importantly, improvements in a field walking test, the shuttle walk, were related to changes in quadriceps strength. Troosters and colleagues extended this concept by randomising patients who had been admitted with acute exacerbation of COPD either to usual care or to receive additional resistance training; the training group avoided exacerbation-related strength loss although, probably due to the sample size, the six-minute walk distance failed to achieve a significant difference between the two groups.18 Interestingly in a subset who consented to biopsy, myostatin was noted to be down regulated in the training group.
Neuromuscular training
Ultimately the unilateral training experiment performed in 2007 was underpowered, and so recently a larger three-way study has been completed to compare unilateral repetitive stimulation, pulmonary rehabilitation and usual care in stable outpatients; the results will be available in late 2011. In the interim, one study has compared the addition of electrical neuromusclular stimulation (NMES) to pulmonary rehabilitation against rehabilitation alone. This study was limited by its small sample size but, nevertheless, suggested that compared with rehabilitation alone conferred benefits judged by walking performance, dyspnoea and quadriceps strength.19
Non-invasive ventilation
Regrettably no big study has emerged in the area of non-invasive ventilation so the consensus remains that this is an experimental adjunctive therapy most likely to offer benefits in patients with the greatest pulmonary limitation.20
Improving lung mechanics
The use of endobronchial valves to reduce ventilation in the areas of the lung with the most emphysema has been previously reported21,22; in a follow-up to that study it was found that patients in whom atelectasis had been induced had significantly better long-term survival.23 Also, in the interim, a multicentre randomised study evaluated the success of endobronchial valves in the treatment of heterogenous emphysema.24 Taken as a group, modest but significant improvements were seen in FEV1 and the six-minute walk distance although this came at the cost of more exacerbations. A post hoc analysis suggested that the benefits were greatest in those with the most heterogenous disease (as expected) and those in whom the fissures were intact: this group, of course, is also the group most likely to achieve atelecasis since they do not have, in the main, collateral ventilation.25
The majority of patients willing to undergo an invasive procedure do, in fact, have evidence of collateral ventilation and in these patients valve implantation is less successful, as indeed is classical lung volume surgery. An alternative approach is to create a passage which allows air to exit the study. One large randomised placebo controlled trial (the EASE study) is ongoing but yet to report. Nevertheless, animal and explanted lung studies suggest that this approach is promising, provided the fenestrations remain patent which is not universally the case in dogs even if a drug eluting device is used.26
An alternative approach therefore is to create a direct connection through the chest wall, a transthoracic pneumonostomy. A proof of concept study has been performed in explanted lung and then in six patients with COPD. In these patients, a mean increase in FEV1 was shown although this was not accompanied by an increase in walking distance.27 Further studies are ongoing but it is believed that this approach may be useful for a minority of patients.
Conclusion
The most important changes to have occurred in the past five years are the recognition that COPD, perhaps because of its multisystem nature, is a different problem to solve and one which has defeated the efforts of individual pharmaceutical companies or universities. The future lies in larger collaborative ventures. Members of our group are participating in three public private partnerships aimed at tackling COPD (the EU IMI, the capability cluster initiative and the MRC-ABPI consortium) and it is to be hoped that this novel way of working can overcome the problems posed.
Funding
The NIHR Respiratory Biomedical Research Unit at the Royal Brompton Hospital and Imperial College part fund Professor Polkey's salary. He discloses receiving payment for advisory boards or lecturing from GlaxoSmithKline (GSK), AstraZeneca (AZ), Chiesi, Novartis, PortAero and Broncus and that his institution holds or has recently held on his behalf as PI grants for COPD studies or for consultancy from GSK, AZ, PortAero, Novartis and the EU-IMI Proactive initiative.
References
- 1.Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease. Clin Med 2006; 6: 190–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349: 1498–504 [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA 2005; 294: 1255–9 [DOI] [PubMed] [Google Scholar]
- 4.Man WD, Soliman MG, Gearing J, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 562–7 10.1164/rccm.200302-162OC [DOI] [PubMed] [Google Scholar]
- 5.Pepin V, Saey D, Whittom F, LeBlanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1517–22 10.1164/rccm.200507-1037OC [DOI] [PubMed] [Google Scholar]
- 6.Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36: 81–8 10.1183/09031936.00104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkinson NS, Polkey MI. Does physical inactivity cause chronic obstructive pulmonary disease? Clin Sci (Lond) 2010; 118: 565–72 [DOI] [PubMed] [Google Scholar]
- 8.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J 2009; 33: 262–72 10.1183/09031936.00024608 [DOI] [PubMed] [Google Scholar]
- 9.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62: 115–20 10.1136/thx.2006.062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swallow EB, Barreiro E, Gosker H, et al. Quadriceps muscle strength in scoliosis. Eur Respir J 2009; 34: 1429–35 10.1183/09031936.00074008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreiro E, Schols AM, Polkey MI, et al. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 2008; 63: 100–7 10.1136/thx.2007.078030 [DOI] [PubMed] [Google Scholar]
- 12.Remels AH, Gosker HR, Schrauwen P, et al. TNF-α impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 2010 Aug 31 doi: 10.1096/fj.09-150714. [DOI] [PubMed] [Google Scholar]
- 13.Doucet M, Russell AP, Leger B, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176: 261–9 10.1164/rccm.200605-704OC [DOI] [PubMed] [Google Scholar]
- 14.Vogiatzis I, Simoes DC, Stratakos G, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J 2010; 36: 301–10 10.1183/09031936.00112909 [DOI] [PubMed] [Google Scholar]
- 15.Man WDC, Natanek SA, Riddoch-Contreras J, et al. Quadriceps myostatin expresssion in COPD. Eur Respir J 2010; 36: 686–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004; 329: 1209. 10.1136/bmj.38258.662720.3A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65: 423–8 10.1136/thx.2009.124164 [DOI] [PubMed] [Google Scholar]
- 18.Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181: 1072–7 10.1164/rccm.200908-1203OC [DOI] [PubMed] [Google Scholar]
- 19.Vivodtzev I, Pepin JL, Vottero G, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006; 129: 1540–8 10.1378/chest.129.6.1540 [DOI] [PubMed] [Google Scholar]
- 20.Corner E, Garrod R. Does the addition of non-invasive ventilation during pulmonary rehabilitation in patients with chronic obstructive pulmonary disease augment patient outcome in exercise tolerance? A literature review. Physiother Res Int 2010; 15: 5–15 10.1002/pri.451 [DOI] [PubMed] [Google Scholar]
- 21.Toma TP, Hopkinson N, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003; 361: 931–3 10.1016/S0140-6736(03)12762-6 [DOI] [PubMed] [Google Scholar]
- 22.Hopkinson NS, Toma TP, Hansell DM, et al. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med 2005; 171: 453–60 10.1164/rccm.200407-961OC [DOI] [PubMed] [Google Scholar]
- 23.Hopkinson NS, Kemp SV, Toma TP, et al. Atelectasis after bronchoscopic lung volume reduction for COPD is associated with improved survival. Eur Respir J 2011; 37: 1346–51 [DOI] [PubMed] [Google Scholar]
- 24.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233–44 10.1056/NEJMoa0900928 [DOI] [PubMed] [Google Scholar]
- 25.Higuchi T, Reed A, Oto T, et al. Relation of nterlobar collaterals to radiological heterogeneity in severe emphysema. Thorax 2006; 61: 409–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choong CK, Phan L, Massetti P, et al. Prolongation of patency of airway bypass stents with use of drug-eluting stents. J Thorac Cardiovasc Surg 2006; 131: 60–4 10.1016/j.jtcvs.2005.07.057 [DOI] [PubMed] [Google Scholar]
- 27.Moore AJ, Cetti E, Haj-Yahia S, et al. Unilateral extrapulmonary airway bypass in advanced emphysema. Ann Thorac Surg 2010; 89: 899–906 10.1016/j.athoracsur.2009.10.067 [DOI] [PubMed] [Google Scholar]