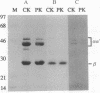
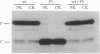
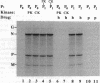
Abstract
We have previously shown that phosphorylation of vesicular stomatitis virus (VSV) phosphoprotein P by cellular protein kinase activity is an essential prerequisite for its transcriptional function. We have now purified this protein kinase by monitoring its ability to phosphorylate bacterially expressed, unphosphorylated P protein. Biochemical studies showed that the kinase is indistinguishable from casein kinase II, a ubiquitous cyclic AMP-independent protein kinase present in a wide variety of eukaryotic cells and tissues. Functional VSV transcription could be reconstituted with viral L protein, N-RNA template, and P protein phosphorylated by either purified cellular protein kinase or purified casein kinase II. The unusual role of casein kinase II in the transcription process of a nonsegmented negative-strand RNA virus would have important implications in host-virus interactions and antiviral therapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Barik S. Gene expression of vesicular stomatitis virus genome RNA. Virology. 1992 Jun;188(2):417–428. doi: 10.1016/0042-6822(92)90495-b. [DOI] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Cloning and expression of the vesicular stomatitis virus phosphoprotein gene in Escherichia coli: analysis of phosphorylation status versus transcriptional activity. J Virol. 1991 Apr;65(4):1719–1726. doi: 10.1128/jvi.65.4.1719-1726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Sequential phosphorylation of the phosphoprotein of vesicular stomatitis virus by cellular and viral protein kinases is essential for transcription activation. J Virol. 1992 Feb;66(2):1109–1118. doi: 10.1128/jvi.66.2.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Ghosh B., Whalen W., Lazinski D., Das A. An antitermination protein engages the elongating transcription apparatus at a promoter-proximal recognition site. Cell. 1987 Sep 11;50(6):885–899. doi: 10.1016/0092-8674(87)90515-0. [DOI] [PubMed] [Google Scholar]
- Beckes J. D., Perrault J. Two distinct protein kinase activities in vesicular stomatitis virions phosphorylate the NS transcription factor. Virology. 1991 Sep;184(1):383–386. doi: 10.1016/0042-6822(91)90854-5. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. NH2-terminal acidic region of the phosphoprotein of vesicular stomatitis virus can be functionally replaced by tubulin. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7977–7981. doi: 10.1073/pnas.85.21.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Huang A. S. Distribution of phosphoserine, phosphothreonine and phosphotyrosine in proteins of vesicular stomatitis virus. Virology. 1981 Jan 30;108(2):510–514. doi: 10.1016/0042-6822(81)90459-1. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Camici M., Lawrence J. C., Jr, Roach P. J. Multiple phosphorylation of rabbit skeletal muscle glycogen synthase. Evidence for interactions among phosphorylation sites and the resolution of electrophoretically distinct forms of the subunit. J Biol Chem. 1983 Sep 10;258(17):10702–10709. [PubMed] [Google Scholar]
- Gill D. S., Banerjee A. K. Vesicular stomatitis virus NS proteins: structural similarity without extensive sequence homology. J Virol. 1985 Jul;55(1):60–66. doi: 10.1128/jvi.55.1.60-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D. C., Haley B. E., Lesnaw J. A. Identification and characterization of serine/threonine protein kinase activity intrinsic to the L protein of vesicular stomatitis virus New Jersey. J Gen Virol. 1992 Jan;73(Pt 1):67–75. doi: 10.1099/0022-1317-73-1-67. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979 Feb 10;254(3):762–768. [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Constitutively phosphorylated residues in the NS protein of vesicular stomatitis virus. J Biol Chem. 1985 Jul 25;260(15):8990–8995. [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massey D. M., Deans N., Lenard J. Phosphorylation of NS protein by vesicular stomatitis virus nucleocapsids: lack of effect during RNA synthesis and separation of kinase from L protein. J Virol. 1990 Jul;64(7):3259–3264. doi: 10.1128/jvi.64.7.3259-3264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Decker K., Amtmann E., Sauer G. Inhibition of the phosphorylation of the regulatory non-structural protein of vesicular stomatitis virus by an antiviral xanthate compound. J Gen Virol. 1987 Dec;68(Pt 12):3045–3056. doi: 10.1099/0022-1317-68-12-3045. [DOI] [PubMed] [Google Scholar]
- Takacs A. M., Perrine K. G., Barik S., Banerjee A. K. Alteration of specific amino acid residues in the acidic domain I of VSV phosphoprotein (P) converts a GAL4-P(I) hybrid into a transcriptional activator. New Biol. 1991 Jun;3(6):581–591. [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]