ABSTRACT
In this clinical audit, we assessed retrospectively the current practice of respiratory physicians with respect to interferon gamma (IFNγ) release assay (IGRA) testing for tuberculosis (TB), as recommended by the 2011 National Institute of Health and Care Excellence (NICE) guidelines for the diagnosis and management of TB. All IGRAs requested by respiratory physicians over a 3-year period were identified retrospectively, and both results and clinical indications analysed. Of the total number of IGRAs carried out, 90% formed part of investigations of suspected active TB. However, 89% of the patients had not had a documented Mantoux test and human immunodeficiency virus (HIV) status was unclear in the 35.2% of patients treated for active TB. Of patients with chest X-rays suggestive of TB, 92.3% were treated for active TB. Of the patients under the age of 35 with reactive IGRAs, 84.6% were treated for active or latent TB and 15.4% had justifiable reasons for not receiving chemoprophylaxis. Based on the results of our audit, IGRAs are commonly being utilised for the investigation of active TB, which is contrary to current guidance.
KEY WORDS: Interferon gamma release assays, tuberculous antigens, tuberculosis, chemoprophylaxis
Introduction
Interferon gamma (IFNγ) release assays (IGRA) are T cell-based assays that detect the release of IFNγ in response to specific tuberculous antigens.1 They were first approved by the US Food and Drug Administration (FDA) in 2001 and have since come into widespread use.2 T-spot.TB tests and QuantiFERON-TB Gold are the major types of commercially available IGRA in clinical use for the detection of tuberculosis (TB).3 In March 2011, the National Institute of Health and Care Excellence (NICE) released new guidelines on the diagnosis and management of TB in the UK. These guidelines were an update on the previous 2006 UK guidelines on TB. One notable change was the incorporation of IGRAs into the algorithm for diagnosis of latent TB.4
Historically, the diagnosis of latent TB has been based on the tuberculin skin test (TST). However, this presented some problems because TST has a significant false positive rate owing to cross-reactivity with other mycobacterial species.5 In addition, the sensitivity of TST is limited in immunocompromised individuals owing to anergy, and there are also problems with consistency in the interpretation of TST results.6 IGRAs are more sensitive and specific compared with TSTs in detecting latent TB.7 In addition, previous vaccination with Bacillus Calmette–Guérin (BCG) does not affect the results. However, a major limitation to the use of the IGRAs is their inability to distinguish latent TB from active TB.8 Therefore, NICE does not recommend IGRA as part of the algorithm for the diagnosis of suspected active TB. Other investigations, including chest X-rays and sputum cultures, must be utilised, in addition to clinical judgement, to make a diagnosis of active TB.4
The aim of this audit was to assess current practice at the University Hospitals Birmingham (UHB) NHS Trust with respect to the context of IGRA use by respiratory physicians and to compare this practice with the 2011 NICE guidelines.
Methods
This was a retrospective study using data that were collected from the microbiology laboratory at UHB. The TB service at UHB is a generalist one with all chest physicians potentially seeing TB cases. UHB does not treat children and there is a immigrant-screening programme at the Trust. All IGRA assays requested by consultant chest physicians from January 2008 until August 2012 were identified from the computerised records of the microbiology department. Further data on demographics, investigations and treatment were obtained via the hospital clinical information portal and clinical notes at the UHB by HRM. Data and reason for IGRAs were discussed by DRT, HRM and RDT to form a consensus upon the reason why the IGRA had been carried out (investigation of patients with symptoms and signs suggestive of active TB, or screening for latent TB, eg before starting anti-tumour necrosis factor (TNF) therapy). Analysis of data was done using Microsoft Excel software.
Results
Demographics
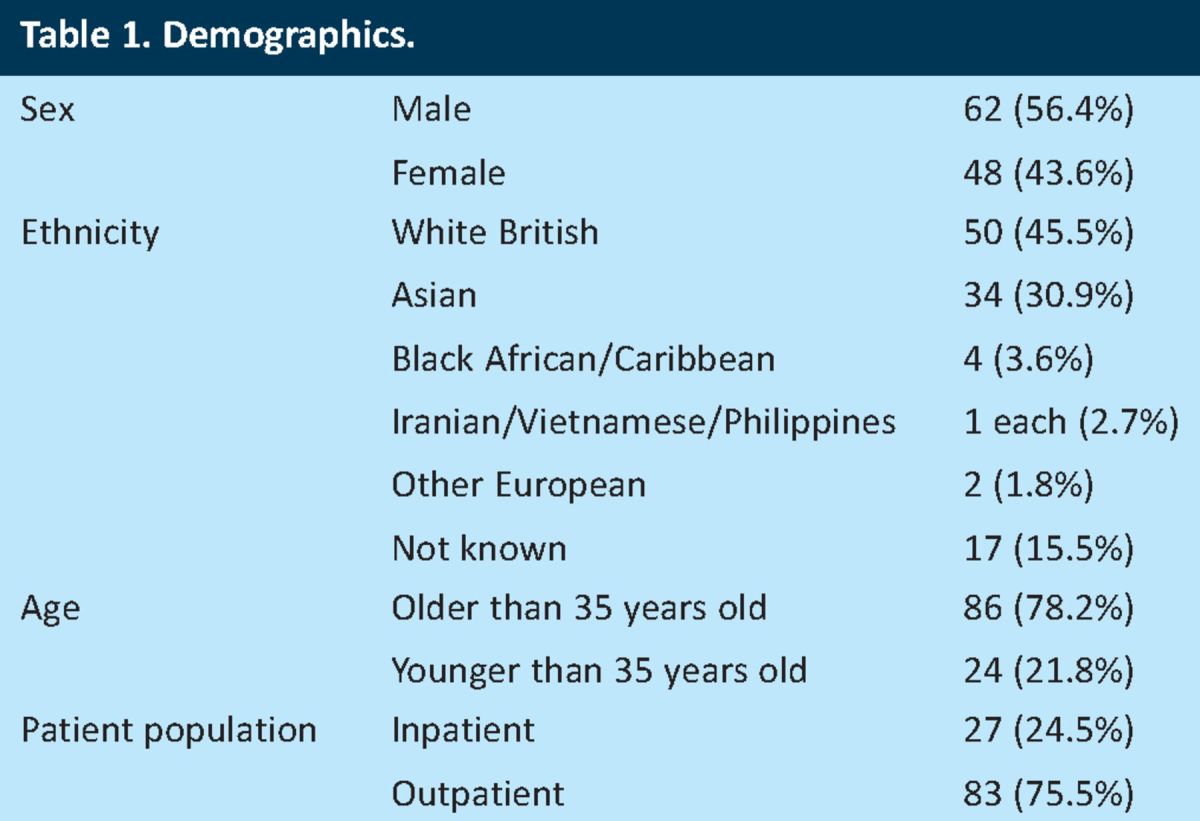
In total, 110 IGRA assay results were identified from 110 patients, and all were included in the audit. Patient demographics are outlined in Table 1. There were slightly more males than females (56.4% vs 43.6%), with the white British and Asian ethnic groups comprising a significant proportion of the study population (45.5% and 30.9%, respectively). The patients were stratified by age, in accordance with the recommendation by NICE that patients with reactive IGRAs below the age of 35 should be treated for latent TB4. Most patients were seen in the outpatient clinics.
Table 1.
Demographics.
Indication for IGRA
The NICE guidelines advocate the use of the IGRA for the diagnosis of latent TB only.4 The reasons for requesting IGRAs in the study population during the study period are summarised in Table 2, with the results of the tests shown in Table 3.
Table 2.
Reason for requesting an interferon gamma release assay.
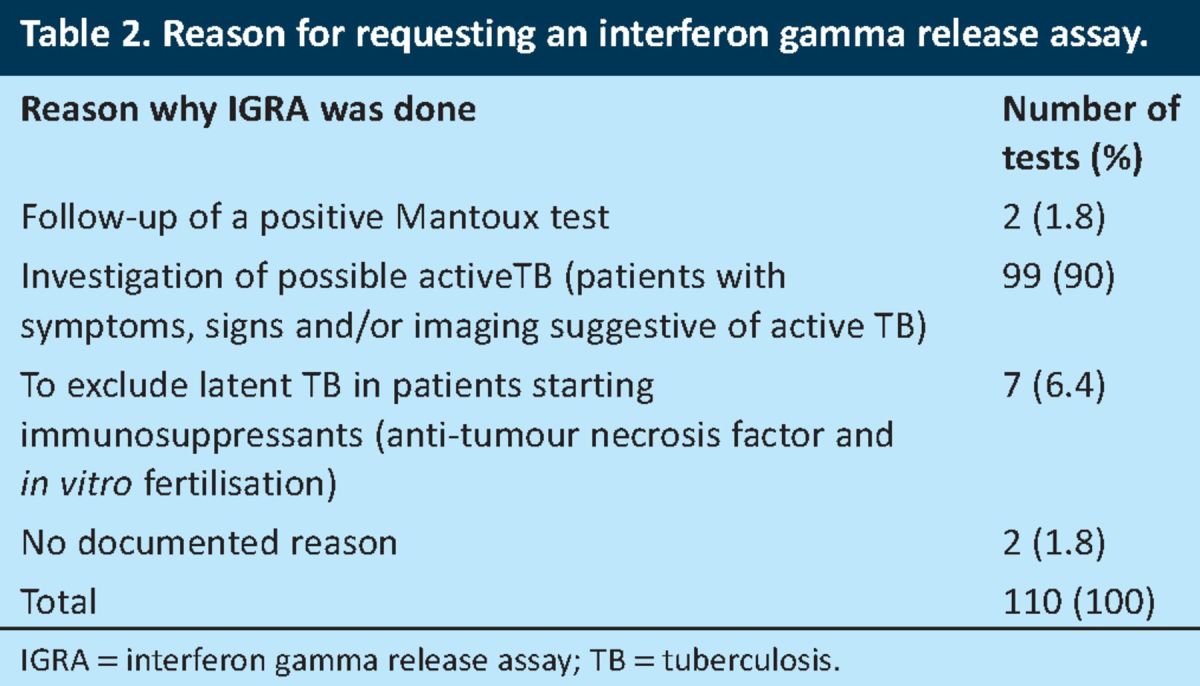
Table 3.
Interferon gamma release assay results by age.
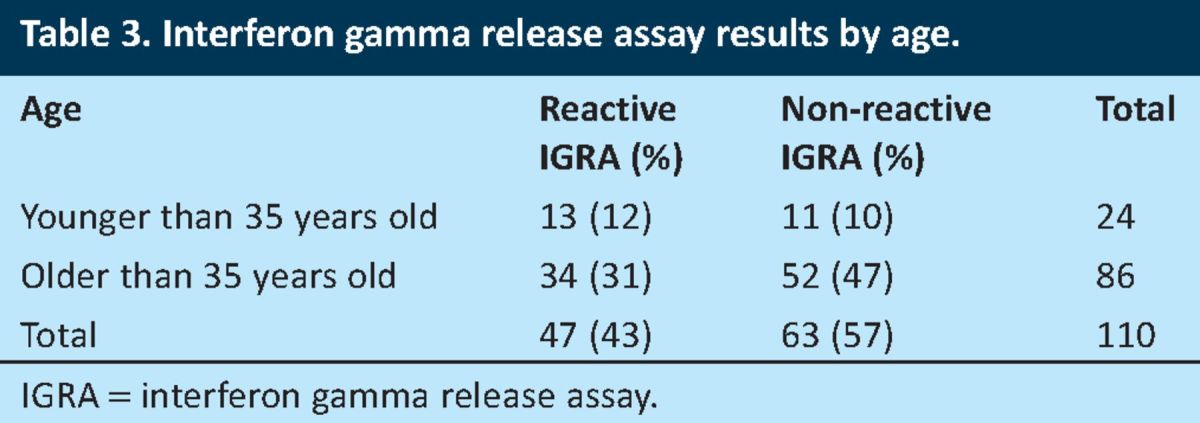
As illustrated in Table 3, reactive IGRAs were obtained in 47 (43%) of the patients included in the study sample, out of which 13 (12% of the entire sample population) were younger than 35 years of age.
Compliance with NICE guidelines on tuberculosis
Compliance with Mantoux testing. According to the NICE guidelines, IGRAs are only advised if the Mantoux test is positive or less reliable.4 Only two of the patients had the IGRAs done as a follow-up of a positive Mantoux test (Table 2). However, some patients were noted in their clinic documentation to have had a Mantoux test at some point during their management. The number of patients with a documented Mantoux test is detailed in Table 4.
Table 4.
Results of Mantoux tests in the study population.

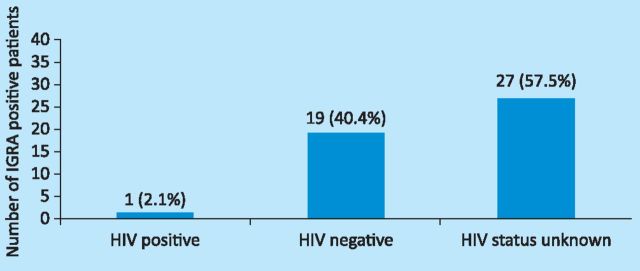
Compliance with HIV testing. The NICE guidelines on TB advise further that patients with human immunodeficiency virus (HIV) and prior BCG vaccination of any age who are strongly Mantoux positive or IGRA positive should be treated for latent TB.4 The HIV status of the patients with reactive IGRAs is shown in Fig 1. Only one (2.1%) of the 47 patients with a reactive IGRA was HIV positive. However, evidence of the HIV status of most patients with reactive IGRAs (57.5%) could not be determined from the electronic or paper records.
Fig 1.
HIV status of patients with reactive interferon gamma release assays (IGRA).
HIV status in the proportion of patients treated for active tuberculosis
In total, 34 of the 110 patients reviewed in this audit were eventually treated for active TB. One of these 34 patients (3%) was HIV positive, 21 (61.8%) were HIV negative and the HIV status was unknown in 12 patients (35.2%).
Compliance with chest X-rays. Chest X-rays are recommended by NICE for patients with reactive IGRAs to exclude active TB.4 In total, 45 (95.7%) of the 47 patients with reactive IGRAs had a chest X-ray. The two patients (4.3%) who did not have chest X-rays had alternative appropriate investigations (CT scan of thorax and lymph node biopsy).
Compliance with additional investigations. Of the 110 patients included in this study, 26 had chest X-ray features that were suggestive of TB. The NICE guidelines advocate sputum microscopy and culture in patients with chest X-ray findings that are indicative of TB.4 It was not clear from the notes whether the patients who did not have sputum microscopy and culture were expectorating (induced sputum is not available at UHB). However, the NICE guidelines advise bronchoscopy and bronchoalveolar lavage (BAL) if spontaneously produced sputum is unavailable. The breakdown of the 26 patients with chest X-rays suggestive of TB who went on to have sputum microscopy and culture and/or a bronchoscopy and lavage is shown in Table 5. All 26 patients who had adequate investigations underwent attempts to obtain culture samples.
Table 5.
Number of patients with chest X-rays suggestive of tuberculosis that had a sputum microscopy and culture and/or a bronchoscopy and lavage.
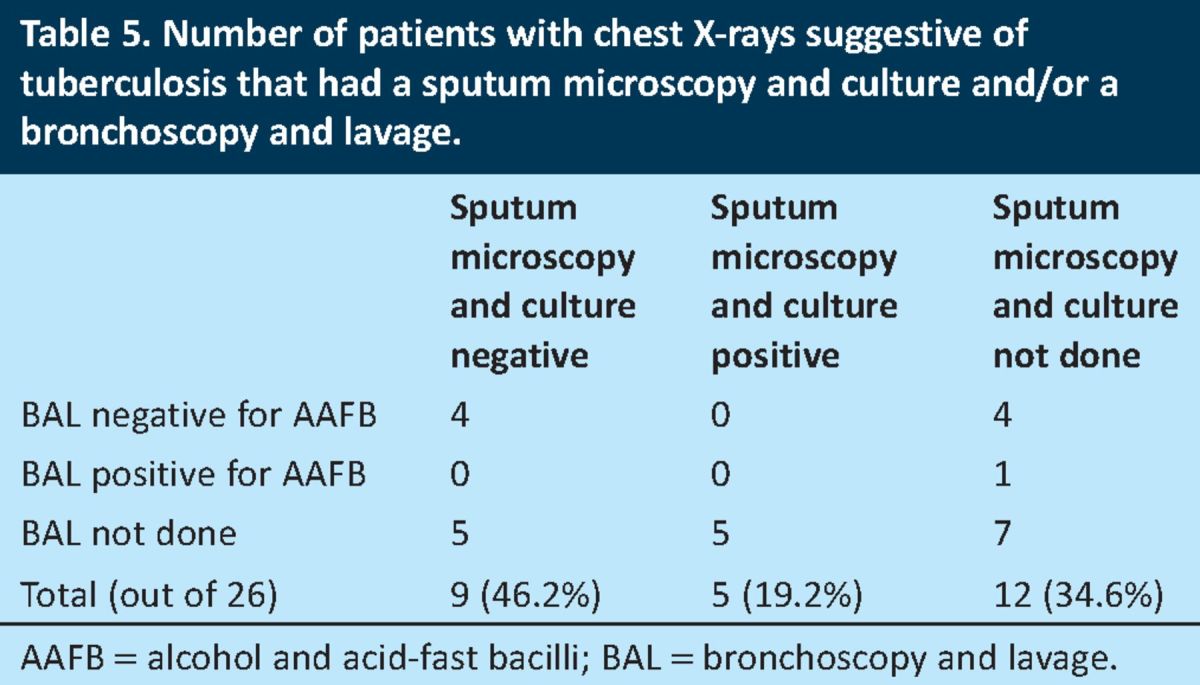
Four of the patients who were sputum microscopy and culture negative also had a negative bronchoscopy and lavage. One of these patients was managed as a case of active TB in view of the high clinical suspicion and history of contact with a patient with active TB. A second patient was found on CT scan of thorax to have miliary TB and was also managed as a case of active TB.
There were five patients with negative sputum tests who did not have a bronchoscopy and lavage. However, in four of these patients, other investigations had been done that included pleural fluid cultures in two patients, one pleural biopsy and one computed tomography (CT) scan of thorax that confirmed miliary TB.
A sputum microscopy and culture was not performed in seven patients who also did not have a bronchoscopy and lavage. However, in all seven patients, there had been additional investigations, including lymph node biopsies in three patients and pleural biopsies in four patients.
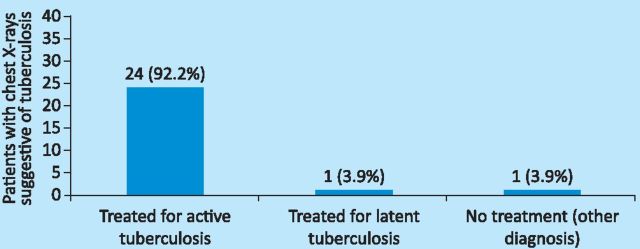
Compliance with the management of active TB. The NICE guidelines propose that patients with chest X-ray appearances that are suggestive of active TB should have further diagnostic investigations, including a sputum microscopy and culture. If these are positive or the patient has clinical signs and symptoms consistent with a diagnosis of TB, then treatment should start immediately.4 The subsequent management of the 26 patients with chest X-rays suggestive of TB is shown in Fig 2.
Fig 2.
Management of patients with chest X-rays suggestive of tuberculosis.
In total, 24 (92.2%) of the patients with chest X-ray features suggestive of TB were treated for active TB; one was treated for latent TB and one was later found to have a parapneumonic effusion.
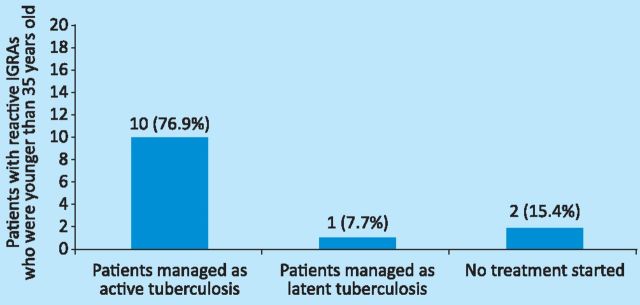
Compliance with the management of latent TB. NICE recommends that treatment of latent TB is considered in patients who are younger than 35 years of age with prior BCG vaccination and are either strongly Mantoux positive or IGRA positive.4 Of the 13 patients (85%) who were younger than 35 years of age and had reactive IGRAs (Table 3), 11 had no record of a Mantoux test being done, and two of the 13 patients (15%) had positive Mantoux tests. Both patients were eventually treated for active TB. The management of all 13 patients who were younger than 35 years of age and had reactive IGRAs is shown in Fig 3.
Fig 3.
Management of patients with reactive IGRA who were younger than 35 years old. IGRA = interferon gamma release assays.
One of the two patients who were not managed as either active or latent TB had been visiting Birmingham at the time, and was referred to a local clinic for subsequent follow-up and management. The second patient was pregnant and a clinical decision was made not to start chemoprophylaxis.
Discussion
Our data suggest that 90% of the IGRAs reviewed in this audit are being utilised mainly for the investigation of patients with suspected active TB, against current guidelines. Only 1.8% had been done as a follow-up of a positive Mantoux test and, in a further 1.8% of the patients, there was no clear indication given for the assay. In total, 6.4% of the patients had the test conducted to exclude latent TB in view of the risk of reactivation of TB when anti-TNF agents were started.9
Although patient ethnicity is not included in the NICE algorithm for the diagnosis and management of latent TB4, TB is well known to follow an ethnic and geographic trend and is more common among foreign-born individuals.10,11 It was observed that the ethnicity of some patients included in the study population was not properly documented (15.5%). Information about patients’ ethnicity is important in the analysis of the potential risk of antibiotic resistance. Furthermore, this omission could impede any larger epidemiological studies in the future.
In this audit, the proportion of patients who had a documented Mantoux result was low (10.9%) and there were no clear reasons given in the patients’ notes about the reason for this omission. Furthermore, a high proportion (84.6%) of the patients below the age of 35 years who had a positive IGRA did not have any record of a Mantoux test being done, thus making it impossible to assess which patients met all the NICE guidance criteria for the treatment of latent TB.
On review of the compliance with HIV testing, the results showed that 57.5% of the patients with reactive IGRAs did not have their HIV status documented. Furthermore, 35.2% of the patients who were treated for active TB did not have their HIV status reported. It is possible that HIV status had been established elsewhere, but nonetheless it would have been expected for this fact to be included in the medical records. Given that TB is an acquired immunodeficiency -syndrome (AIDS)-defining illness,12,13 it is important that a patient who is being treated for active TB should be screened for HIV -infection.
There was high compliance with the NICE guidelines recommendation that patients with reactive IGRAs should have a follow-up chest X-ray to exclude active TB. In total, 45 of the 47 patients (95.7%) with reactive IGRAs had had a chest X-ray and the remaining two patients had other forms of imaging. There were 26 patients with chest X-ray findings that were suggestive of TB, all of whom had appropriate additional investigations in line with the NICE guidelines.
Most patients who had chest X-ray features suggestive of TB, received treatment for active TB (92.3%), thus demonstrating a high compliance with the NICE guidelines. The compliance with the NICE guidelines on the management of latent TB was also high, with 11 of the 13 patients with reactive IGRAs who were younger than 35 years of age (84.6%) being treated for either active or latent TB.
Conclusion
IGRAs are T cell-based assays that have recently been incorporated into the NICE guidelines for the diagnosis of latent TB. The aim of this audit was to assess the use of IGRAs at the UHB and compare this with the recently published NICE guidelines. The results of the audit indicated that IGRAs are being utilised for the investigation of active TB outside of their licence and current guidelines. The ethnicity of the patients was not always clearly documented and, in some cases, Mantoux tests were not done or results not stated. Several patients who had been treated for active TB did not have HIV tests done.
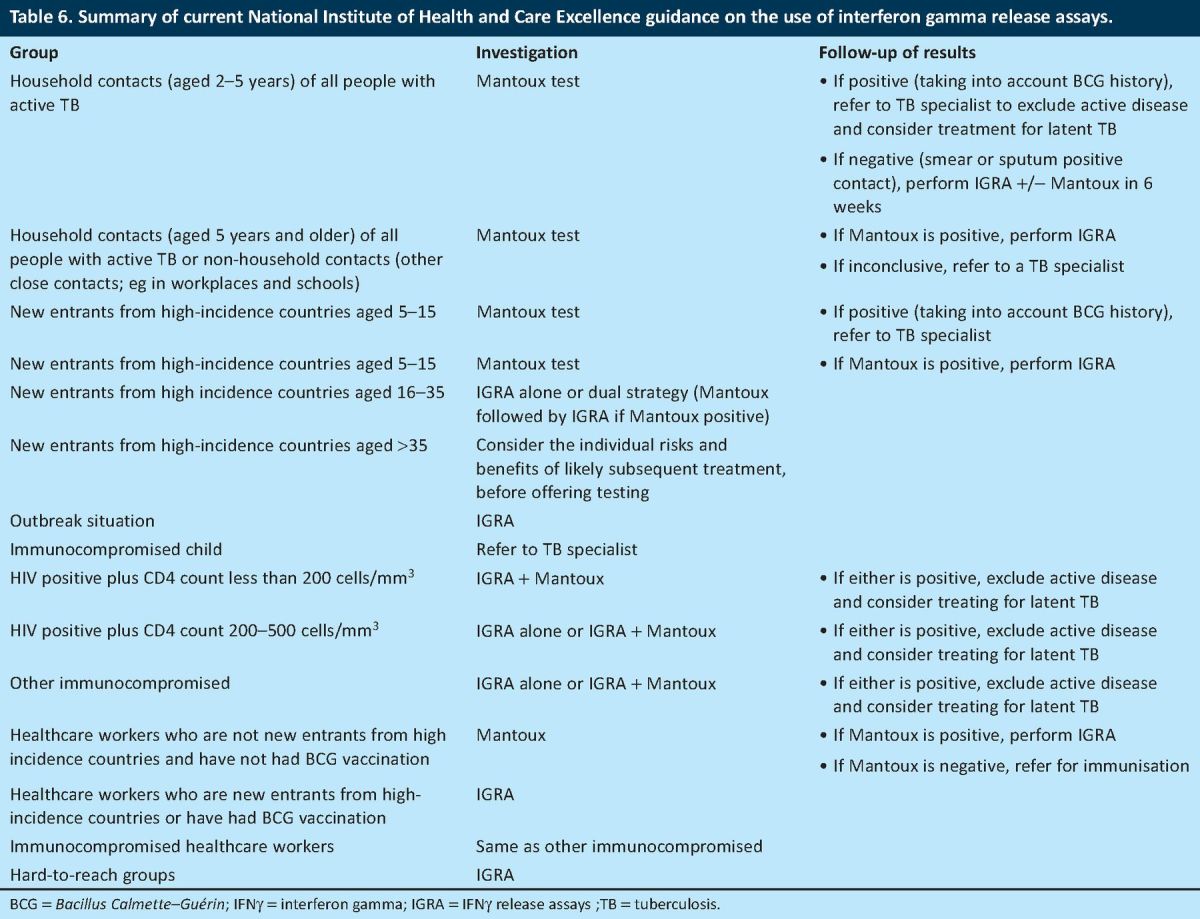
A positive finding of this audit was that all the patients with reactive IGRAs had gone on to have chest X-rays or other appropriate imaging. All the patients with chest X-ray findings that were suggestive of TB underwent further investigations, including any of a sputum microscopy and culture, BAL, pleural fluid microscopy and culture, lymph node biopsy or pleural biopsy. Furthermore, every patient had received appropriate treatment for either active or latent TB. The patients with reactive IGRAs for whom treatment for latent TB had been recommended according to the NICE guidelines were also managed properly in line with the current guidelines (Table 6).
Table 6.
Summary of current National Institute of Health and Care Excellence guidance on the use of interferon gamma release assays.
In conclusion, it is recommended that:
IGRAs should be used for the diagnosis of latent TB in line with current guidelines
the ethnicity of all patients should be clearly documented
the results of the Mantoux tests should be clearly documented and reasons stated whenever the test is not done
all patients with active TB should be screened for HIV infection.
these indicators of quality of use will be re-audited subsequently.
Acknowledgements
We thank Deborah Mortiboy, consultant microbiologist at the UHB NHS Trust, for assistance.
References
- 1.Lalvani A, Millington KA. T cell interferon gamma release assays: can we do better? Eur Resp J 2008;32:1428-30. 10.1183/09031936.00148308 [DOI] [PubMed] [Google Scholar]
- 2.Cyndee M, Tomford JW, Gordon SM. Interferon gamma release assays: better than tuberculin skin testing? Cleve Clin J Med 2010;77:606-11. 10.3949/ccjm.77a.09112 [DOI] [PubMed] [Google Scholar]
- 3.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon gamma release assays for detecting active TB. A meta-analysis. Chest 2010;137:952-68. 10.1378/chest.09-2350 [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Health and Care Excellence Tuberculosis: Clinical Diagnosis and Management of tuberculosis and measures for its prevention and control. London: NICE, 2011. www.nice.org.uk/guidance/index.jsp?action=byID&o=13422 [Assessed 5 June 2013]. [PubMed] [Google Scholar]
- 5.Lalvani A. Diagnosing tuberculosis infection in the 21st century. New tools to tackle an old enemy. Chest 2007;131:1898-906. 10.1378/chest.06-2471 [DOI] [PubMed] [Google Scholar]
- 6.Ozuah PO, Burton W, Lerro KA, et al. Assessing the validity of tuberculin test readings by trained professionals and patients. Chest 1999;116:104-6. 10.1378/chest.116.1.104 [DOI] [PubMed] [Google Scholar]
- 7.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection. Areas of uncertainty and recommendations for research. Ann Intern Med 2007;146:688. 10.7326/0003-4819-146-5-200703060-00006 [DOI] [PubMed] [Google Scholar]
- 8.Lange C, Pai M, Drobniewski F, Miglion GB. Interferon gamma release assays for the diagnosis of active tuberculosis: sensible or silly? Eur Resp J 2009;33:1240-53. 10.1183/09031936.00019709 [DOI] [PubMed] [Google Scholar]
- 9.Liote H, Liote F. Role for interferon gamma release assays in latent tuberculosis screening before TNF-a antagonist therapy. Joint Bone Spine 2011;78:352-57. 10.1016/j.jbspin.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Miramontes R, Pratt R, Price S, et al. Trends in Tuberculosis – United States 2011. MMWR Morb Mortal Wkly Rep 2012;61:11. [PubMed] [Google Scholar]
- 11.Welshman J. Tuberculosis and ethnicity in England and Wales 1950–1970. Sociol Health Illness 2000;22:858-82. 10.1111/1467-9566.00234 [DOI] [Google Scholar]
- 12.Posnansky MC, Coker R, Skinner C, et al. HIV positive patients first presenting with an AIDS-defining illness: characteristics and survival. BMJ 1995;311:156. 10.1136/bmj.311.6998.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.British HIV Association, British Association of Sexual Health and HIV, and British Infection Society. UK National Guidelines for HIV testing 2008. London: BHIVA, 2008. www.bhiva.org/documents/Guidelines/Testing/GlinesHIVTest08.pdf [Accessed 5 June 2013]. [Google Scholar]