Abstract
Lithium (Li) may cause multiple endocrinopathies, including hypercalcaemia, thyroid dysfunction and nephrogenic diabetes insipidus (NDI), but rarely in the same patient. The management of NDI remains a challenge. We report on a patient on long-term Li who had simultaneous NDI (paired serum and urine samples had abnormal osmolalities, typical of NDI, and treatment with parenteral desmopressin failed to affect urinary volume and serum osmolality), ‘destructive’ thyroiditis (hyperthyroidism, absent radioiodine uptake and absent thyrotrophin receptor antibodies) and primary hyperparathyroidism (compatible biochemistry, urine calcium excluding ‘set point’ anomalies and hypocalciuric hypercalcaemia, and normal parathyroid imaging). The thyroiditis resolved spontaneously and hypercalcaemia responded to reduction of Li dose. The NDI was unresponsive to amiloride, thiazides and ibuprofen in combination. However, urine output was reduced by 50% when a high dose of oral desmopressin was given. We conclude that Li-induced multiple endocrinopathy remains rare and, although NDI is difficult to manage, high dose oral desmopressin should be tried when other medications fail.
Introduction
Lithium (Li), an effective anti-psychotic medication,1 occasionally causes endocrine dysfunction. Some endocrine complications are difficult to manage and may persist after Li withdrawal. These endocrinopathies usually occur alone, but can rarely occur at the same time, increasing the complexity of management.2,3
The mechanisms causing some forms of endocrine dysfunction are clear. Hypothyroidism, which occurs in 20–30% of those affected, is caused primarily by prevention of intraglandular thyroid hormone release.3 Hypercalcaemia results from decreased parathyroid gland sensitivity to calcium, set point shift of the calcium–parathyroid hormone (PTH) curve to the right and associated hyperparathyroidism.4 However, the mechanisms causing nephrogenic diabetes insipidus (NDI) are unclear.5
Some endocrine dysfunctions are easy to manage, but the management of others, particularly NDI, remains unsatisfactory. Withdrawal of Li may help, but this can cause worsening of -psychiatric symptoms and some endocrinopathies remain intractable after long-term Li therapy.
Clinical presentation
A 37-year-old patient with paranoid schizophrenia had been treated with Li for over 10 years. He was referred to the endocrinology department due to abnormalities found during routine testing (free T4 27 pmol/l [reference range 9.2–24.5], TSH <0.01 mU/l [0.2–4.5] and sodium 150 mmol/l [133–146]). He had a long history of polyuria (approximately 15 l/day), excessive thirst (leading to excessive fluid intake) and nocturia (passing copious amounts of urine every 2 hours). On examination, he was eupituitary and euthyoid with no goitre. The results of the investigations carried out are shown in Tables 1 and 2. The results confirmed:
Table 1.
Results of investigations at presentation and review. At presentation, there was evidence of inappropriately low urine osmolality combined with high serum osmolality accompanying hypernatraemia, which was suggestive of diabetes insipidus. Also, hypercalcaemia and inappropriately ‘normal’ PTH levels combined with urine calcium levels were inconsistent with familial hypocalciuric hypercalcaemia or calcium ‘set point’ anomalies; hypercalcaemia normalised on Li dose reduction. Finally, thyroid antibody negative for hyperthyroidism subsided spontaneously.
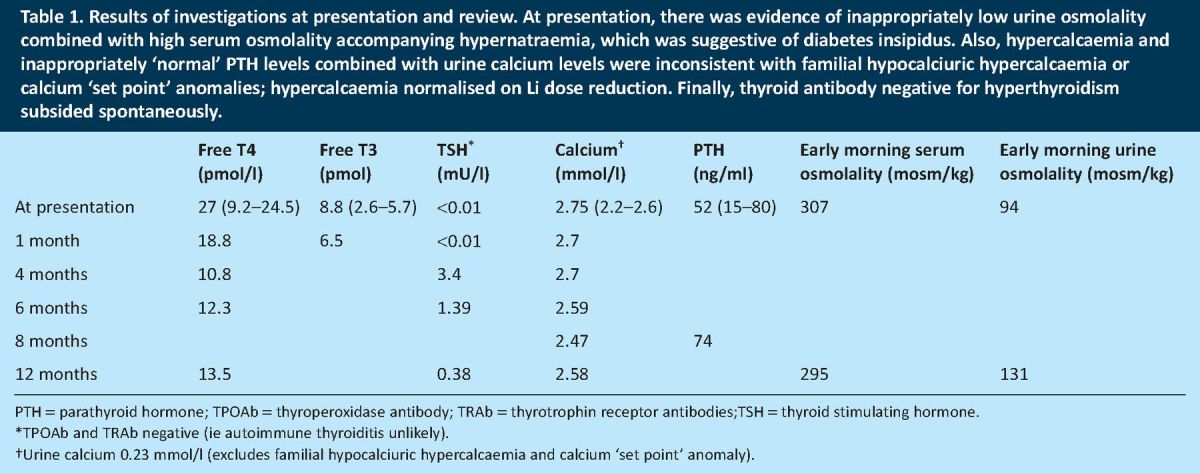
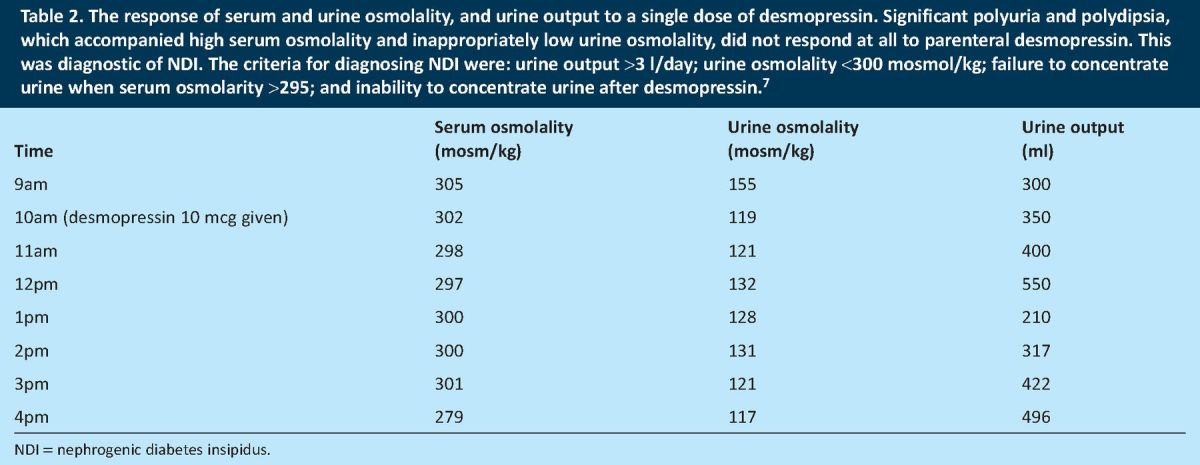
Table 2.
The response of serum and urine osmolality, and urine output to a single dose of desmopressin. Significant polyuria and polydipsia, which accompanied high serum osmolality and inappropriately low urine osmolality, did not respond at all to parenteral desmopressin. This was diagnostic of NDI. The criteria for diagnosing NDI were: urine output >3 l/day; urine osmolality <300 mosmol/kg; failure to concentrate urine when serum osmolarity >295; and inability to concentrate urine after desmopressin.7
NDI – consisting of hypernatraemia, elevated serum -osmolality and inappropriately low urine osmolality (paired samples), and lack of response of urinary volume and osmolality to parenteral desmopressin
‘destructive’ thyroiditis – including raised thyroid hormones and suppressed thyroid stimulating hormone (TSH) (which then spontaneously returned to normal), very low radioactive iodine uptake (RAI) (Fig 1), absent thyrotrophin receptor antibodies (TRAb) and normal thyroid ultrasound imaging
primary hyperparathyroidism (adenoma or multiple gland hyperplasia) – including high serum calcium and inappropriately ‘normal’ PTH, urinary calcium excluding a calcium ‘set point’ anomaly and familial hypocalciuric hypercalcemia (FHH), and no focal parathyroid abnormality on ultrasound and Sestamibi scans.
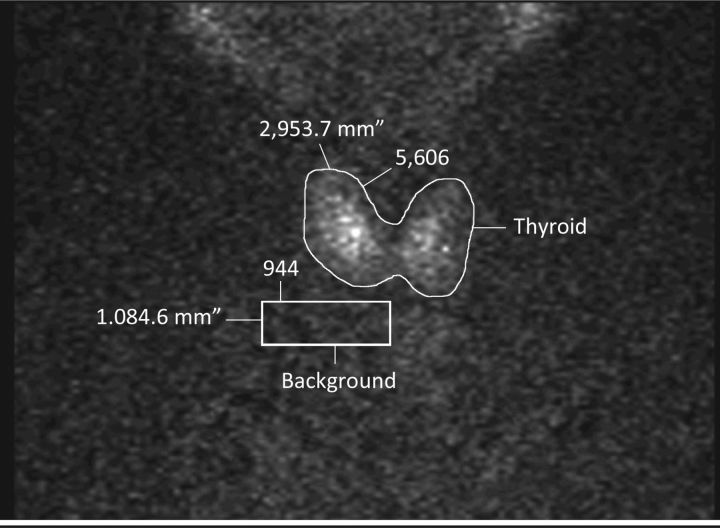
Fig 1.
Radioactive iodine uptake scan showing very low thyroid uptake. Thryoid uptake here is 0.5% (a normal range would be 5–35%). Low RAI uptake and hyperthyroidism were indicative of Li-induced ‘destructive thyroiditis’. Li = lithium; RAI = radioactive iodine.
Abdominal CT scans showed non-obstructive dilatation of the renal pelvicalyceal systems and hydroureters bilaterally (Fig 2).
Fig 2.
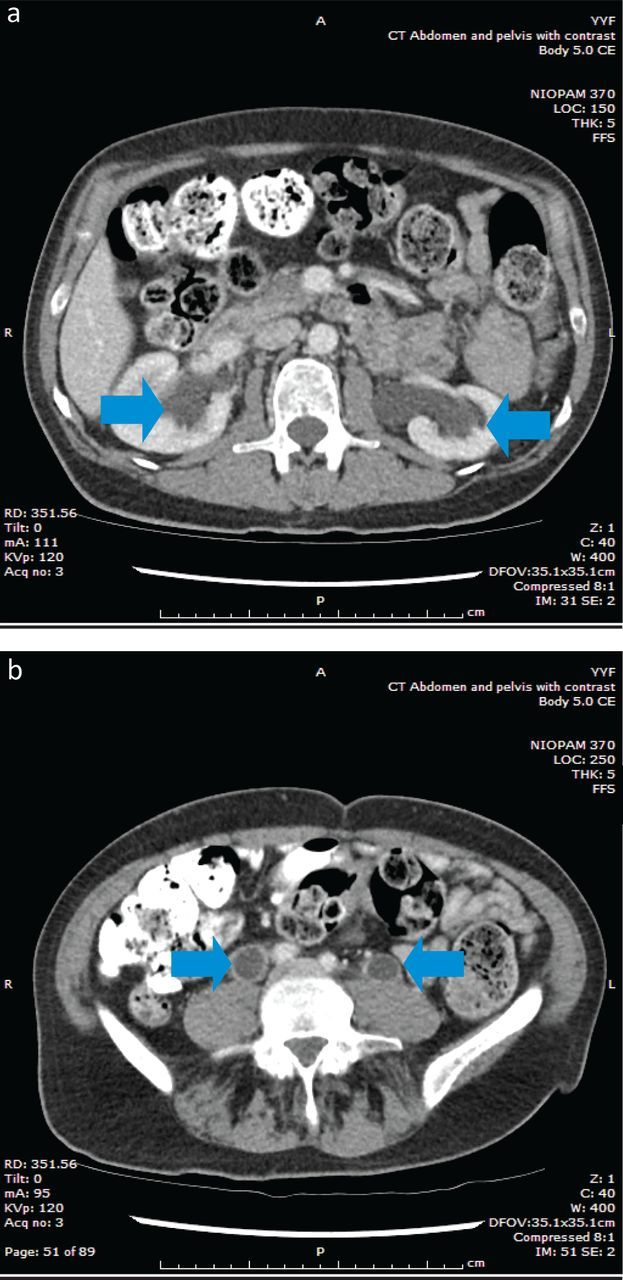
Abdominal CT scans showing bilateral dilated pelvicalyceal systems and hydroureters. Abdominal CT scans show dilated pelvicalyceal systems (a) and hydroureters (b) bilaterally. These are non-obstructive and occur due to large volume of renal and ureteric urine flow. CT = computed tomography.
Lithium and endocrine dysfunction
Nephrogenic diabetes insipidus
NDI occurs in up to 40% of Li-treated patients, but subtle abnormalities of urinary concentration may occur in an even larger group.
Urinary concentration requires the coordinated function of several mechanisms. Arginine vasopressin (AVP) is released by the posterior pituitary gland when serum osmolality rises. AVP binds to V2 receptors in the renal tubular cells resulting in insertion of aquaporin 2 (AQP2) water channels and increased AQP2 expression. Water moves from the tubular lumen to the interstitium through these water channels.
Li enters renal tubular cells through epithelial sodium channels (ENaC) and causes a significant reduction of AQP2 expression (ie, it causes NDI). Amiloride blocks ENaC-mediated Li entry into renal cells and ameliorates this effect.6 Li-induced NDI may become irreversible in some subjects after treatment for more than 2–10 years.
Thyroid dysfunction
Goitre and hypothyroidism – Li prevents pre-formed thyroid hormone (TH) release. The resultant rise in TSH leads to goitre formation and hypothyroidism. There is an increased risk of this in females and those with positive thyroperoxidase antibody (TPOAb).
Hyperthyroidism – Hyperthyroidism occurs through follicular destruction, TH release and escape from the inhibition of thyroid hormone release, as described above. The effect of Li on GD remains unclear but toxic multinodular goitre has been reported.
Hypercalcaemia
Li causes hypercalcaemia through several mechanisms: altering parathyroid calcium-sensing receptor set point for parathyroid hormone (PTH) inhibition;4 blocking enzymes that suppress PTH gene transcription – the resulting uninhibited PTH gene transcription leads to parathyroid hyperplasia or adenoma -formation; increasing calcium reabsorption through higher PTH levels; and blocking calcium influx into cells.
Li may unmask primary hyperparathyroidism in those harbouring adenomas (within 2 years) and cause four-gland hyperplasia in others (after 10–12 years of starting treatment).
Clinical progress
The effect of Li withdrawal
NDI persists or recurs after Li withdrawal. The effects on -calcium homeostasis are less clear. There is sparse consistent evidence supporting normalisation of calcium and PTH on Li withdrawal – parathyroidectomy is needed in some. The effect of Li withdrawal on thyroid dysfunction is also variable and unpredictable.
Clinical progress
The patient's thyroiditis resolved spontaneously and hypercalcaemia normalised (following dose reduction of Li from 1.2 g/day to 500 mg/day), suggesting multiple gland hyperplasia than an autonomous adenoma (Table 1).
Management of NDI
Amiloride was given at a dose of 10 mg/day. We were reluctant to introduce thiazides because the patient had hypercalcaemia and mild renal dysfunction during amiloride treatment. However, bendroflumethiazide 2.5 mg/day was given when both had normalised, but with no effect. The addition of 400 mg ibuprofen twice daily was not helpful either.
The use of desmopressin, the synthetic analogue of AVP, in NDI is paradoxical and seems counterintuitive. Early studies showed partial response to supraphysiological doses alone or in combination with indomethacin in Li-induced NDI and X-linked NDI in children.7 It is speculated that partial AVP resistance in the renal tubules in Li-induced NDI is overcome by high-dose desmopressin therapy. We gave our patient 600 μg oral desmopressin three times per day. His symptoms improved considerably with almost 50% reduction in urine output (7.9 l/day compared to 15 l/day). His nocturia became significantly reduced and sleep patterns were very much improved.
Therapeutic challenges
Li withrawal – The clinical utility of Li withdrawal in NDI following long-term therapy remains doubtful. However, dose reduction may have resulted in normalisation of serum calcium in our subject.
Thiazide diuretics – We were reluctant to use thiazide diuretics with Li because of Li potentiation, renal dysfunction (with amiloride) and worsening of hypercalcaemia.
Pharmacological doses of desmopressin – Despite the paucity of published evidence, therapeutic doses of desmopressin were given to the patient. Informed consent from the patient and his parents was given, and the patient was closely monitored throughout treatment.
Conclusions
Over 800,000 Li prescriptions were dispensed in 2008 in England, costing more than £1.45 million.8 The management of some forms of Li-induced endocrine dysfunction is challenging. Our patient had multiple simultaneous Li-induced endocrinopathies, which is rare, and his NDI remains only partially responsive to therapy. The addition of high dose oral desmopressin has improved his symptoms, reduced urine output by nearly 50% and improved his quality of life. His destructive -thyroiditis was self limiting and reduction of Li dose may have normalised his hypercalcaemia.
References
- 1.Muller-Oerlinghausen B, Berghofer A, Bauer M. Bipolar disorder. Lancet 2002;359:241–7. 10.1016/S0140-6736(02)07450-0 [DOI] [PubMed] [Google Scholar]
- 2.Fridman A, Nguyen Q, Plummer E. A case of lithium-induced polyendocrinopathy and thyroid storm. Endocrinologist 2010;20:131–3. 10.1097/TEN.0b013e3181dfdb14 [DOI] [Google Scholar]
- 3.Lazarus JH. Lithium and the thyroid. Best Pract Res Clin Encodcrinol Metab 2009;23:723–33. 10.1016/j.beem.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Haden ST, Stoll AL, McCormick S, et al. Alterations in parathyroid dynamics in lithium-treated subjects. J Clin Endocrinol Metab 1997;82:2844–8. 10.1210/jc.82.9.2844 [DOI] [PubMed] [Google Scholar]
- 5.Khairallah W, Fawaz A, Brown EM, Fuleihan GE. Hypercalcemia and diabetes insipidus in a patient previously treated with lithium. Nat Clin Prac Nephrol 2007;3:397–404. 10.1038/ncpneph0525 [DOI] [PubMed] [Google Scholar]
- 6.Bedford JJ, Weggery S, Ellis G, et al. Lithium-induced nephrogenic -diabetes insipidus: renal effects of amiloride. Clin J Am Soc Nephrol 2008;3:1324–31. 10.2215/CJN.01640408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstock RS, Moses AM. Desmopressin and indomethacin therapy for nephrogenic diabetes insipidus in patients receiving lithium carbonate. South Med J 1990;83:1475–7. 10.1097/00007611-199012000-00026 [DOI] [PubMed] [Google Scholar]
- 8.Gerrett D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ 2010;341:c6258. 10.1136/bmj.c6258 [DOI] [PubMed] [Google Scholar]