ABSTRACT
Non-melanoma skin cancer (NMSC) comprises basal cell carcinoma (BCC) and squamous cell carcinoma, together with a host of rare tumours. NMSC is the commonest malignancy among Caucasians and its incidence continues to rise annually. Exposure to UV radiation initiates approximately 90% of NMSC, causing malignant transformation of keratinocytes and suppression of the inflammatory response. Risk factors include sun exposure and immunosuppression. There are several subtypes of BCC, although histological overlap is common. Surgery has traditionally been regarded as the ‘gold-standard’ treatment, offering excellent cure rates and cosmetic results. Other treatment modalities include physical destruction (radiotherapy, curettage and cautery, and cryotherapy), chemical destruction (photodynamic therapy and topical 5-flurouracil) and immunomodulatory therapy (topical imiquimod). The recent development of novel hedgehog pathway inhibitors for high-risk BCC (including oral vismodegib and sonidegib) may represent a paradigm shift towards medical management of NMSC.
KEYWORDS: Non-melanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, surgery, dermatology
Key points
Non-melanoma skin cancer (NMSC) is the commonest form of cancer among Caucasians and its incidence is increasing annually.
Basal cell carcinoma (BCC) and squamous cell carcinoma comprise the majority of NMSCs.
Surgery is regarded as the ‘gold standard’ of treatment with high success rates.
Several less invasive, topically applied therapies are available for treatment of some cases of BCC and pre-malignant lesions.
The development of novel systemic therapies (such as vismodegib) for advanced cases of BCC may herald a paradigm shift from surgical to medical management of NMSCs.
Introduction
Non-melanoma skin cancer (NMSC) is the most common malignancy among Caucasians.1 NMSC principally comprises basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), but includes a host of rarer skin tumours.
NMSC incidence is rising annually and is projected to cost the NHS £180 million by 2020.2 Although mortality rates are low, significant morbidity results as lesions commonly occur on sun-exposed sites such as the face.1 Risk factors for non-melanoma skin cancer are fair skin, genetic susceptibility, living in areas of high ultraviolet (UV) radiation exposure, previous occurrence, age and male sex.3
Epidemiology
In 2011, over 102,000 cases of NMSC were diagnosed in the UK, 75% of which were BCCs.4 Factors accounting for the rising incidence are the ageing population and greater recreational exposure to UV radiation due to foreign travel and use of sunbeds. There is clear regional variation; the highest rates are in the South West (121.29 and 33.02 per 100,000 person-years for BCC and SCC respectively) and the lowest in London (0.24 and 14.98 per 100,000 person years for BCC and SCC respectively).1 BCC occurs most frequently and accounts for three-quarters of registered cases of NMSC.4
Pathogenesis
Exposure to UV radiation initiates approximately 90% of NMSCs.5 Both BCC and SCC result from the malignant transformation of keratinocytes and suppression of the cutaneous inflammatory response.6 Iatrogenic immunosuppression following organ transplantation results in a greater incidence of NMSC, with reversal of the BCC: SCC ratio and occurrence of more aggressive SCC. Caucasian transplant recipients have an increased risk of 65–250 times for SCC and 10–16 times for BCC.7 HIV infection also confers an approximately 2-fold greater risk of NMSC.8 Complex interplay between various factors, including dose of UV radiation, age, skin type, degree and chronicity, contribute to the pathogenesis of NMSC in immunosuppression.7 Physicians should be cognisant that immunosuppressive medications, such as ciclosporin, azathioprine and mycophenolate mofetil, given for indications other than organ transplantation (including inflammatory bowel disease, rheumatological disease, vasculitides and atopic dermatitis) will result in a predisposition to NMSC. Patients taking immunosuppressants should use sun protective measures, undertake regular self-surveillance and report suspicious lesions to their clinician for urgent evaluation.
Presentation
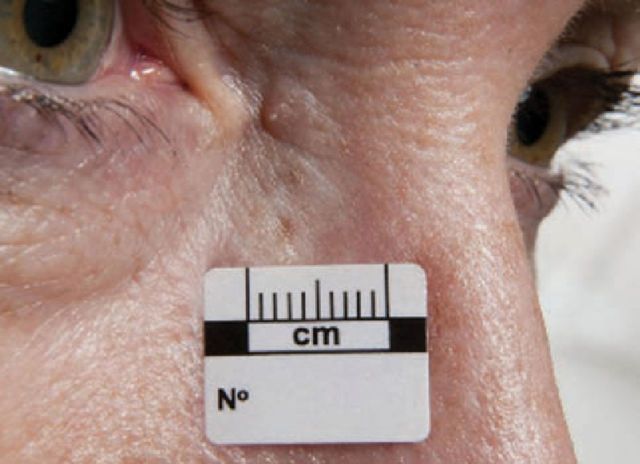
BCC occurs mainly on sun-exposed sites, with 80% appearing on the head and neck and 15% on the trunk.9 Lesions are slow growing and metastases are rare, but local invasion and destruction of surrounding structures can occur if lesions are untreated. There are several histological subtypes of BCC. The classical nodular subtype presents as a pink, pearly papule with overlying telangiectasia and a rolled edge and may have central ulceration (rodent ulcer).
Superficial BCCs are slowly enlarging erythematous plaques, which are more frequent on the trunk and can mimic psoriasis, Bowen’s disease or discoid eczema.10 Morphoeic BCCs are more invasive and present at a late stage due to the non-specific appearance of pale, poorly demarcated plaques.10 There is a high degree of overlap among the subtypes of BCC and a mixed histological picture is most commonly encountered.11
SCC may present as ulcers or indurated keratinising lesions on sun-exposed sites. SCCs may develop from pre-malignant lesions, actinic keratoses (AKs) and Bowen’s disease, which is also termed SCC in situ. AKs are a marker of UV-damaged skin and progress to invasive SCC in approximately 1–10% of cases.7 Keratoacanthomas are typically nodular, keratinising lesions, evolving over several months with spontaneous involution. They are histologically indistinct from well-differentiated SCCs and excision is therefore essential for all cases.
Management
The British Association of Dermatologists has published guidelines for the management of both BCC and SCC, which are under regular review.12,13 Historically, surgery has been the mainstay (‘gold standard’) of treatment. Other treatments can be broadly categorised as physical destruction, chemical destruction and immunomodulation. The last decade has witnessed the advent of novel systemic therapies for advanced BCC, which may herald a paradigm shift of NMSC treatment to a more medical approach.
Surgery
The aim of surgery is removal of the entire tumour with optimal cosmetic results. Excision of well-defined, low-risk SCCs <2-cm diameter with a 4-mm margin would be expected to remove the primary tumour in 95% of cases.13 Primary BCC is also effectively treated by excision with a 4-mm margin, giving a 5-year recurrence rate of <2%.12 Wider margins or alternative treatment methods are required for larger and poorly differentiated lesions. Mohs micrographic surgery allows for examination of the entire excision margins with staged resection, and provides excellent cure rates for high-risk BCC and SCC.12,13
Physical destruction
Radiotherapy has cure rates of up to 90% reported for SCC, 5-year cure rates of 91.3% for primary BCC and 90.2% for recurrent BCC.13–15 Where tissue preservation is paramount (lip, lower eyelid and inner canthus of the eye), for bony or cartilaginous sites and in patients for whom surgery is contraindicated, radiotherapy is often the treatment of choice.13 Primary BCC and recurrent BCC post-surgery can also be successfully treated, although radiotherapy is contraindicated in recurrent BCC post-radiotherapy and naevoid basal cell carcinoma syndrome.7 Inferior cosmetic results previously made radiotherapy a less attractive option for younger patients, but techniques have improved in recent years.
Some low-grade BCC and SCC can be treated with physically destructive techniques such as curettage and cautery or cryotherapy, but this does not allow for histological evaluation of margins and may give poor cosmetic outcomes.12
Chemical destruction
Over the last two decades, less invasive topically applied treatments have been developed that facilitate chemical destruction of tumours.
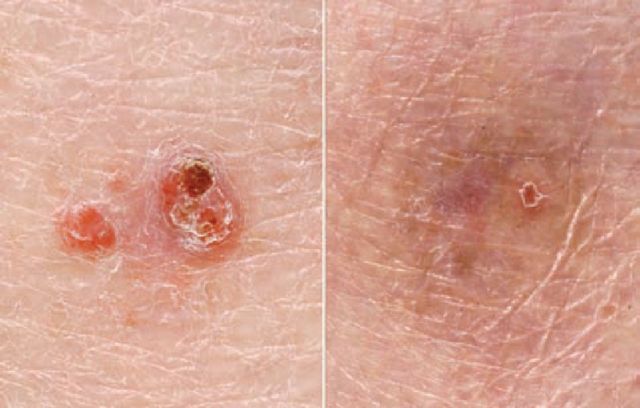
Topical photodynamic therapy (PDT) uses a photosensitising agent (methyl aminolevulinate (MAL) or 5-aminolevulinic acid), activated by a light source. The photosensitiser produces photo-active porphyrins in malignant keratinocytes and illumination then results in the release of reactive oxygen species and free radical formation.16 MAL PDT is licensed for the treatment of BCC in the UK and has shown favourable clinical and cosmetic outcomes in randomised trials in comparison to cryotherapy.12 However, it is not recommended for high-risk tumours unless more effective treatment modalities are inappropriate or refused by the patient. Pain is a further limiting factor. PDT is not approved for SCC, due to the potential for metastases and recurrence, but it is effective in the management of premalignant lesions.16 A specific advantage for AK is the possibility of field treatment of subclinical, photo-damaged skin with excellent cosmetic results. Of note, PDT is less effective in organ transplant recipients on long-term immunosuppression.
5-fluorouracil, which suppresses the enzyme thymidylate synthetase and prevents synthesis of DNA and RNA is also a well-established treatment for small superficial BCCs, as well as the pre-malignant lesions, AKs and Bowen's disease.7 Other chemically destructive topical agents for AKs include diclofenac gel (applied twice daily for three months) and ingenol mebutate gel (applied once daily for only two or three days). Topical agents may invoke an intense inflammatory response, with localised erythema and potential pustulation and ulceration.
Immunomodulatory therapy
Imiquimod, an immune modifier which acts on toll-like receptors, induces production of cytokines and chemokines from dendritic cells and monocytes.12 Topical 5% imiquimod is licensed in Europe for superficial BCC, but has not shown efficacy for nodular BCC or SCC management.12,13
Medical management
Systemic treatment of BCC
Mutations of the tumour suppressor gene PTCH1 have recently been identified as underlying 90% of sporadic BCCs as well as naevoid basal cell carcinoma syndrome (Gorlin syndrome).9PTCH1 aberrations generate signalling errors of transmembrane proteins in the hedgehog pathway, causing failure to suppress signalling of the G-protein-coupled receptor smoothened (SMO).9 Manipulation of the PTCH1 and SMO pathways has formed the premise of recent developments in systemic treatment of BCC.17
Oral vismodegib was the first small molecule inhibitor of SMO in the hedgehog pathway, approved for use in January 2012 for locally advanced and metastatic BCC unsuitable for conventional treatment.17 The SafeTy Events in VIsmodEgib (STEVIE) study is an ongoing international multicentre open-label study, representing the most comprehensive data series regarding safety and efficacy of vismodegib to date.18 Interim results showed response rates comparable to previous studies and progression free survival of 20.2 months for the intention to treat population (n = 496). Frequently occurring and cumulative adverse events (affecting over two-thirds of patients) may limit the tolerability of vismodegib for many patients. These include muscle cramps, taste disturbance, weight loss, fatigue and alopecia.3 Similar class effects are seen with other novel hedgehog pathway inhibitors (eg sonidegib) and the possibility of dose alteration to reduce adverse events is under investigation.18 Oral sonidegib 200 mg daily has shown a promising risk-benefit profile for advanced BCC in a recent multi-centre randomised double-blind phase-II trial.19 The antifungal agent itraconazole inhibits the hedgehog pathway via a separate mechanism and is another promising candidate drug being investigated as a systemic treatment for BCC.20
Conclusions
The burgeoning incidence of NMSC will continue to present a huge financial and logistical challenge to the NHS, particularly as our population ages. The advent of novel medical therapies is an exciting development, but their long-term efficacy is yet to be determined. Awareness of these common malignancies remains essential for all medical specialties.
Fig 1.
Basal cell carcinoma. Reproduced with permission of Salford Royal NHS Foundation Trust.
Fig 2.
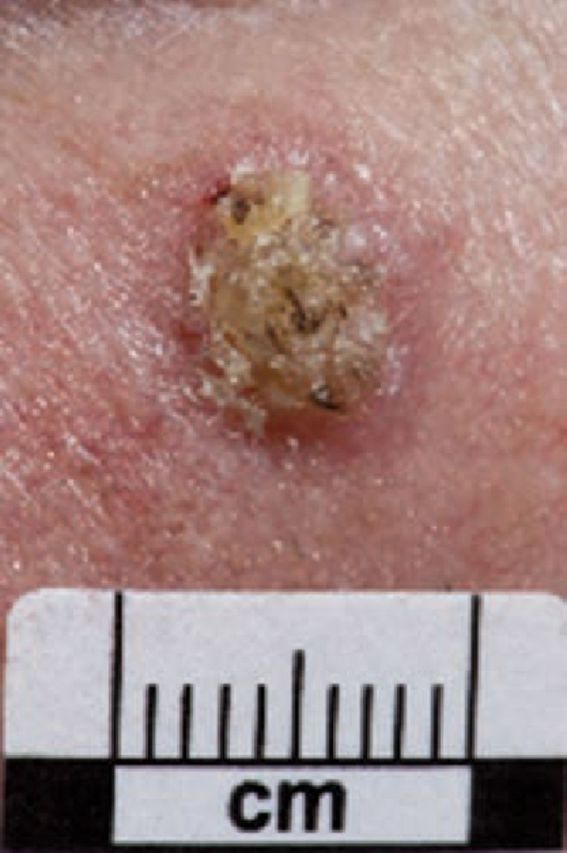
Squamous cell carcinoma. Reproduced with permission of Salford Royal NHS Foundation Trust.
Fig 3.
Superficial BCC (a) before and (b) after treatment with photodynamic therapy. Reproduced with permission of Salford Royal NHS Foundation Trust.
Conflicts of interest
JTL has accepted honoraria for speaking at meetings by Leo, Galderma, Almirall, Astellas and GlaxoSmithKline.
Acknowledgements
We thank the clinical photography department of Salford Royal NHS Foundation Trust for providing the clinical images. Copyright is retained by the department.
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of non-melanoma skin cancer. Br J Dermatol 2012;166:1069–80. [DOI] [PubMed] [Google Scholar]
- 2.Leigh IM. Progress in skin cancer: the U.K. experience. Br J Dermatol 2014;171:443–5. [DOI] [PubMed] [Google Scholar]
- 3.Ali FR, Lear JT. Systemic treatments for basal cell carcinoma (BCC): the advent of dermato-oncology in BCC. Br J Dermatol 2013;169:53–7. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Research UK. Skin cancer statistics. London: CRUK, 2015. Available online at www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/skin-cancer [Accessed 26 November 2015] [Google Scholar]
- 5.Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010;463:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erb P, Jingmin J, Kump E, et al. Apoptosis and pathogenesis of melanoma and non-melanoma skin cancer. Adv Exp Med Biol 2008;624:283–95. [DOI] [PubMed] [Google Scholar]
- 7.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet 2010;375:673–85. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst 2013;105:350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med 2005;353:2262–9. [DOI] [PubMed] [Google Scholar]
- 10.Wong CSM, Strange RC, Lear JT. Basal cell carcinoma. BMJ 2003;327:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma: study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol 1990;23:1118–26. [DOI] [PubMed] [Google Scholar]
- 12.Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35–48. [DOI] [PubMed] [Google Scholar]
- 13.Motley RJ, Preston PW, Lawrence CM. Multi-professional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol 2002;146:18–25 [updated 2009]. [DOI] [PubMed] [Google Scholar]
- 14.Rowe DE, Carroll RJ, Jr Day CL, et al. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol 1989;15:315–28. [DOI] [PubMed] [Google Scholar]
- 15.Rowe DE, Carroll RJ, Day CL. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol 1989;15:424–31. [DOI] [PubMed] [Google Scholar]
- 16.Braathen LR, Szeimies RM, Basset-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. J Am Acad Dermatol 2007;56:125–43. [DOI] [PubMed] [Google Scholar]
- 17.Lear JT. Oral hedgehog-pathway inhibitors for basal-cell carcinoma. N Engl J Med 2012;366:2225–6. [DOI] [PubMed] [Google Scholar]
- 18.Basset-Seguin N, Hauschild A, Grob J. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol 2015;16:729–36. [DOI] [PubMed] [Google Scholar]
- 19.Midgen MR, Guminski A, Gutzmer R. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol 2015;16:716–28. [DOI] [PubMed] [Google Scholar]
- 20.Kim DJ, Kim J, Spaunhurst K. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol 2014;32:745–51. [DOI] [PubMed] [Google Scholar]