To induce tenogenesis of bone marrow-derived mesenchymal stem cells (BMSCs), a stepwise tenogenic differentiation approach was established by first using transforming growth factor-β1 stimulation, followed by combination with connective tissue growth factor. Gene expression analysis showed that this protocol initiated and maintained highly efficient tenogenesis of BMSCs. Tendons treated with induced tenogenic BMSCs had better structural and mechanical properties than controls.
Keywords: Tendon repair, Mesenchymal stem cells, Differentiation, Growth factor
Abstract
Tendon injuries are common and present a clinical challenge, as they often respond poorly to treatment and result in long-term functional impairment. Inferior tendon healing responses are mainly attributed to insufficient or failed tenogenesis. The main objective of this study was to establish an efficient approach to induce tenogenesis of bone marrow-derived mesenchymal stem cells (BMSCs), which are the most common seed cells in tendon tissue engineering. First, representative reported tenogenic growth factors were used as media supplementation to induce BMSC differentiation, and the expression of teno-lineage transcription factors and matrix proteins was compared. We found that transforming growth factor (TGF)-β1 significantly induced teno-lineage-specific gene scleraxis expression and collagen production. TGF-β1 combined with connective tissue growth factor (CTGF) elevated tenomodulin and Egr1 expression at day 7. Hence, a stepwise tenogenic differentiation approach was established by first using TGF-β1 stimulation, followed by combination with CTGF for another 7 days. Gene expression analysis showed that this stepwise protocol initiated and maintained highly efficient tenogenesis of BMSCs. Finally, regarding in situ rat patellar tendon repair, tendons treated with induced tenogenic BMSCs had better structural and mechanical properties than those of the control group, as evidenced by histological scoring, collagen I and tenomodulin immunohistochemical staining, and tendon mechanical testing. Collectively, these findings demonstrate a reliable and practical strategy of inducing tenogenesis of BMSCs for tendon regeneration and may enhance the effectiveness of cell therapy in treating tendon disorders.
Significance
The present study investigated the efficiency of representative tenogenic factors on mesenchymal stem cells’ tenogenic differentiation and established an optimized stepwise tenogenic differentiation approach to commit tendon lineage differentiation for functional tissue regeneration. The reliable tenogenic differentiation approach for stem cells not only serves as a platform for further studies of underlying molecular mechanisms but also can be used to enhance cell therapy outcome in treating tendon disorders and develop novel therapeutics for tendon injury.
Introduction
Tendons are elastic collagenous tissues that connect muscles and bones. The main function of tendon is to transmit forces from muscles to bones for maintenance of normal body movement. Tendon injuries occur frequently during sports and other rigorous activities and are usually associated with significant morbidity and abnormal joint movement [1]. Because of the limited self-repair capacity of tendon tissues, natural tendon healing often gives rise to inferior mechanical properties, and the healed tendon is susceptible to reinjury. To date, functional healing of tendon injuries has been a great challenge. Current treatment is limited and unsatisfying, as the biochemical properties and functions of healed tendon tissue never recover to those of intact tendon [2–5]. Patients often suffer from long-term pain, discomfort, and even disability. Therefore, more effective therapeutic techniques for tendon repair are needed.
Tendon tissue engineering is a most promising approach and opens new possibilities for tendon regeneration. Mesenchymal stem cells (MSCs) are known to be multipotent and self-renewing, and MSCs can be isolated from various tissues and easily cultured. Thus MSCs have been extensively studied and applied in tissue engineering as seed cells for tendon repair. Despite many successful studies showing that MSC application improves tendon healing, there are concerns about the risk of spontaneous chondrogenesis and ectopic bone formation after MSC transplantation in tendon injury models [6]. The chondro-osteogenetic changes in tendon tissues lead to the erroneous deposition of extracellular matrix and contribute to the poor quality of injured tendon.
The induction of MSCs to differentiate into tendon-forming cells in vitro before transplantation may be a useful approach for promoting tendon repair. Various strategies for teno-lineage differentiation of MSCs have been reported, including mechanical stimulation, tenogenic master gene transfection, coculture with tenocytes, physical topography induction, decellularized tendon matrices, and the use of bioactive factors [7–11]. Most of them depend on specialized equipment, are time-consuming, and often yield variable and inconsistent results. Bioactive molecules, especially recombinant growth factors, are readily available, dose controllable, and widely used for potential clinical applications. At present, several growth factors have been reported to be able to induce tenogenic differentiation in MSCs, including connective tissue growth factor (CTGF, also known as CCN2), growth differentiation factor (GDF) family members (GDF-5/bone morphogenetic protein [BMP]-14, GDF-6/BMP-13, and GDF-7/BMP-12), and transforming growth factor (TGF)-β family members (TGF-β1, TGF-β2, TGF-β3, etc.) [12–20]. However, it is difficult to determine which represents the most efficient and effective strategy because of the lack of direct comparative studies under identical conditions. In this study, we investigated the efficiency of representative tenogenic factors on MSC tenogenic differentiation, based on levels of teno-lineage marker expression and collagen production. We also optimized the tenogenic differentiation strategy by stepwise treatment of the selected growth factors. The induced MSCs after in vitro tenogenic differentiation were applied in a tendon injury model to evaluate efficacy for tendon repair compared with MSCs without induction.
Materials and Methods
Isolation and Culture of Rat Fluorescence-Tagged Bone Marrow-Derived MSCs
All experiments were approved by the Animal Research Ethics Committee of the Chinese University of Hong Kong. Four- to six-week-old male green fluorescent protein (GFP)-tagged Sprague-Dawley rats, weighing 250–300 g, were used. The procedure of isolation and culture of rat GFP-tagged bone marrow-derived mesenchymal stem cells (BMSCs) was described previously [21]. The cells were suspended in α-essential Eagle’s medium (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) containing 10% fetal bovine serum (Thermo Fisher Scientific Life Sciences) and 1% penicillin–streptomycin–neomycin (Thermo Fisher Scientific Life Sciences), seeded into T75 flasks (Corning, Corning, NY, http://www.corning.com), and incubated at 37°C in a humidified atmosphere with 5% CO2. MSCs from passage 2 or 3 were used for experiments.
Upon reaching confluence, BMSCs were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) medium alone (Ctrl) or supplemented with (a) 10 ng/ml TGF‐β1 (PeproTech, Rocky Hill, NJ, http://www.peprotech.com), (b) 100 ng/ml BMP-12 (PeproTech), or (c) 100 ng/ml CTGF (PeproTech) and 50 µg/ml ascorbic acid (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), or all resulting combinations, with conditioned medium changed every 3 days. Growth factor concentrations were selected on the basis of previously published reports on tendon differentiation strategies [12, 14, 15, 18].
Quantitative Polymerase Chain Reaction
Total cellular RNA was isolated by lysis in TRIzol (Thermo Fisher Scientific Life Sciences). The expression levels of tendon-specific genes in cells cultured with different media were assessed by quantitative polymerase chain reaction (PCR). PCR was performed using Brilliant SYBR Green QPCR Master Mix (TaKaRa Bio, Shiga, Japan, http://www.takara-bio.com) on a Light Cycler apparatus (ABI 7900HT). The PCR cycling consisted of 40 cycles of amplification of the template DNA, with primer annealing at 60°C. The relative expression level of each target gene was then calculated using the 2−ΔΔCt method. Rpl19 was used as endogenous reference gene. PCR efficiencies of target genes and Rpl19 were approximately equal. Data are presented as fold change relative to the expression level of negative control samples (untreated BMSCs). All primers (Tech Dragon, Hong Kong, People’s Republic of China, http://www.techdragon.com.hk) were designed using primer 5.0 and are summarized in supplemental online Table 1.
Sirius Red Staining
After induction for 7 days, the conditioned medium was removed, and the cells were washed with phosphate-buffered saline. Before Sirius red staining, cells were fixed with 70% ethanol for 30 minutes and washed 3 times. The deposited collagen was stained with 0.1% Sirius red in saturated aqueous solution of picric acid. To quantify the stained nodules, the stain was solubilized with 0.5 ml of 1:1 (vol/vol) 0.1% NaOH and absolute methanol for 30 minutes at room temperature. Solubilized stain (0.1 ml) was transferred to wells of a 96-well plate, and absorbance was measured at 540 nm. Data are presented as mean ± SD, n = 3.
In Vivo Neotendon Formation in Nude Mice
To demonstrate that induced BMSCs can form neotendon in vivo, a nude mouse model was applied. Briefly, after anesthesia, an incision was made on the dorsum, and a subcutaneous pocket was created to expose the posterior midline. The cell sheet formed by 5 × 105 induced BMSCs or 5 × 105 BMSCs in fibrin glue (Beriplast P Combi-Set; CSL Behring, King of Prussia, PA, http://www.cslbehring.com) was sutured to posterior midline at both ends using Ethicon 6-0 suture, and there was tensile strength on the tendon graft with movement. At the end of 4 and 6 weeks (n = 4), the implanted tissues were harvested and subjected to histology for examination of vascularity and collagen fiber alignment.
Animal Model of Patellar Tendon Injury and Repair
Thirty-four Sprague-Dawley male adult rats (8 weeks old, body weight 250–300 g) were used. To create the tendon defect, the central one-third of the patellar tendon (∼1 mm in width) was removed from the distal apex of the patella to the insertion of the tibia tuberosity with two stacked sharp blades according to a well-established protocol from our previous work [12]. The rats were divided into two groups: those treated with (a) BMSCs in fibrin glue and (b) induced BMSC cell sheets. The engineered tendon tissue was placed in the tendon defect and sutured to the patellar bone and tibia tuberosity using Ethicon 6-0. The animals were allowed free cage activity until euthanasia. At weeks 2 and 6 after surgery, 5 animals in each group were killed, and the patellar tendons were harvested for ex vivo examination of the presence of transplanted cells by fluorescence imaging, histology for the examination of cellularity and vascularity of the regenerated tissue, and polarization microscopy for the assessment of collagen fiber alignment, as well as collagen content determination. At week 6, another 7 animals from each group were euthanized, and both contralateral intact and injured patellar tendons were harvested for biomechanical tests.
Immunofluorescence
Briefly, cells were fixed in 4% paraformaldehyde for 10 minutes at room temperature, permeabilized, and blocked for 30 minutes with 1% bovine serum albumin. Fixed cells were washed and incubated with a primary antibody against collagen type I (Abcam, Cambridge, MA, http://www.abcam.com), tenomodulin (Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), or control immunoglobulin G (BD, Franklin Lakes, NJ, http://www.bd.com) at 4°C overnight. Cells were incubated with Alexa Fluor 555-conjugated secondary antibody (Thermo Fisher Scientific Life Sciences) for 2 hours, and nuclei were stained with 4′,6-diamidino-2-phenylindole. For quantification of signal intensity, images were captured with the same gain, offset, magnitude, and exposure time. This was followed by random selection of a minimum of three different images, and intensities were quantified using Image-Pro Plus software as previously reported [9].
Histologic Evaluation and Masson Trichrome Staining
Specimens were immediately fixed in 10% neutral buffered formalin, dehydrated through an alcohol gradient, cleaned, and embedded in paraffin blocks. Histologic sections (5 µm) were prepared using a microtome and stained with hematoxylin and eosin (H&E). In addition, Masson trichrome staining was performed according to standard procedures to examine the general appearance of the collagen fibers. Polarizing microscopy was used to detect mature collagen fibrils.
Immunohistochemistry
Paraffin sections (5 μm) were incubated in antigen retrieval buffer (100 mM Tris and 5% [wt/vol] urea, pH 9.5) at 60°C for 60 minutes for antigen retrieval. Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide in methanol for 10 minutes. Nonspecific protein binding was blocked by incubation with 10% goat serum. After overnight incubation at 4°C with antibodies to collagen type I (Abcam) and tenomodulin (Santa Cruz Biotechnology), sections were incubated with goat antirabbit horseradish peroxidase (HRP)-conjugated or donkey antigoat HRP-conjugated secondary antibody (Santa Cruz Biotechnology) for 2 hours at room temperature. The 3,3′-diaminobenzidine substrate system (DAB; Dako, Glostrup, Denmark) was used for color development. Hematoxylin staining was used to reveal the nuclei.
Mechanical Testing
Mechanical testing was performed using a tension/compression system with Fast-Track software (Model 5543; Instron, Norwood, MA, http://www.instron.us). The hindlimbs were wrapped in gauze soaked in saline and frozen at −80°C for later testing. Before testing, the hindlimbs were gradually thawed to room temperature, and all soft tissue spanning the knee, except for the center of the patellar tendon, were sharply transected. Measurement of the tendon cross-sectional area was performed using aqueous rapid-curing alginate dental impression paste, digital photography, and computerized image analysis as previously reported [22]. The femur-patellar tendon-tibia complex (FPTC) was then rigidly fixed to custom-made clamps. After applying a preload of 0.1 N, each FPTC underwent preconditioning by cyclic elongation of 0–0.5 mm for 20 cycles at 5 mm/min. This was followed by load-to-failure test at an elongation rate of 5 mm/min. The load-elongation behavior of the FPTCs and failure modes were recorded. The structural properties of the FPTC were represented by stiffness (newtons per millimeter), ultimate load (newtons), energy absorbed at failure (millijoules), and stress at failure. For each FPTC, the greatest slope in the linear region of the load-elongation curve over a 0.5-mm elongation interval was used to calculate stiffness.
Statistical Analysis
All quantitative data sets are expressed as mean ± SD. One-way analysis of variance and Student’s t test were performed to assess whether there were statistically significant differences in the results between groups. Values of p < .05 were considered to be statistically significant. The significance level is presented as either p < .05 or p < .01.
Results
Effect of Different Bioactive Factors on BMSCs’ Tenogenic Differentiation
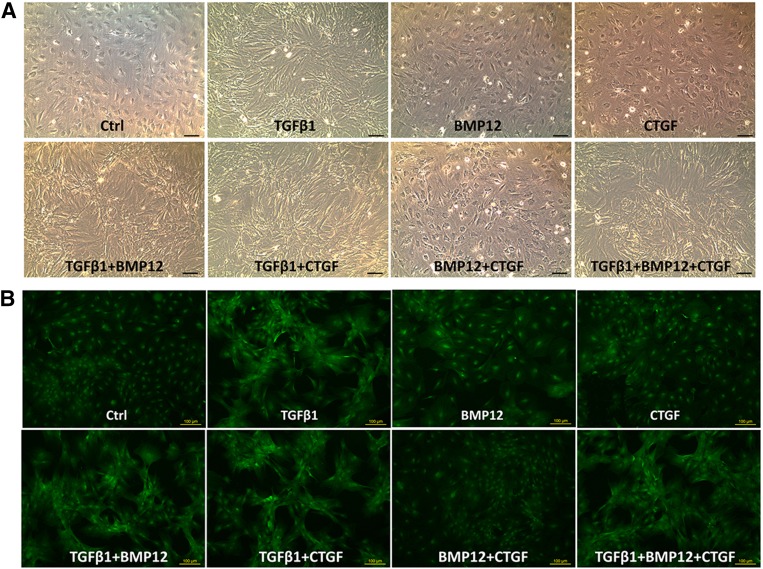
The typical morphology of tendon cells is spindle-like in shape and longitudinally oriented to collagen fiber bundles in tendon tissue. Because the morphologic features are crucial for functional tissue, we observed MSC morphology when cultured in the different conditioned media. It was found that BMSCs elongated notably in the presence of TGF-β1, alone and with with other exogenous factors (combination groups) (Fig. 1A). Under fluorescent microscopy, GFP fluorescence confirmed the cell morphology changes and BMSC growth in clusters under TGF-β1 alone or with other exogenous factors (Fig. 1B).
Figure 1.
Morphologic appearance of BMSCs in culture supplemented with various growth factors. (A): Representative phase-contrast images showing the change of BMSC morphology under different treatments at day 3. (B): GFP fluorescence images showing BMSC growth patterns under different treatments at day 3. Scale bars = 100 µm. Abbreviations: BMP12, bone morphogenetic protein; BMSC, bone marrow-derived mesenchymal stem cell; CTGF, connective tissue growth factor; Ctrl, control; TGFβ1, transforming growth factor β1.
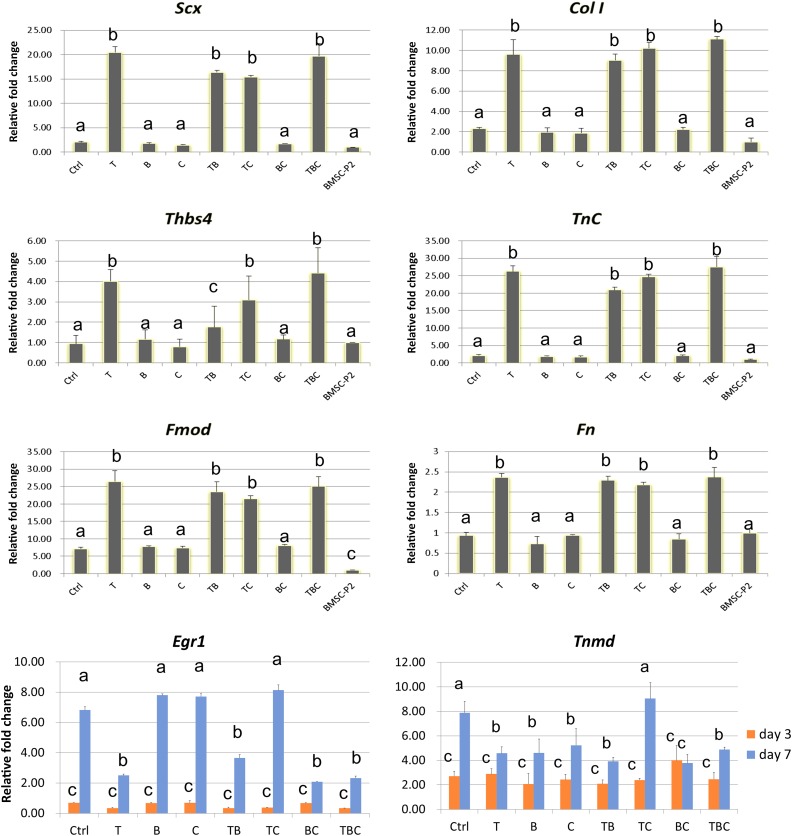
Gene expression was measured in BMSCs (P2) before induction and in BMSC culture in the absence (Ctrl) or presence of exogenous factors. Messenger RNA (mRNA) expression is presented in Figure 2, normalized to BMSCs (P2). Notably, expression of tenogenic gene Scx and tendon matrix genes Col I, Fmod, Fn, TnC, and Thbs4 was significantly increased in the presence of TGF-β1 alone and with other exogenous factors at day 3 (Fig. 2). Treatment with BMP-12 alone, CTGF alone, or their combination did not exhibit any significant difference in teno-lineage gene expression compared to the control group or BMSCs (P2). No significant difference in cell morphology was observed between TGF-β1 alone and with other growth factors. Although expression levels of the other transcription gene Egr1 and tendon specific marker Tnmd at day 3 did not show any significant difference between all 8 groups, Egr1 expression was elevated significantly in BMP12 alone, CTGF alone, and TGF-β1/CTGF groups at day 7 compared with day 3 (Fig. 2). The greatest increase in Tnmd gene expression was observed in the presence of TGF-β1/CTGF (Fig. 2).
Figure 2.
Tenogenic marker gene expression trends in BMSCs cultured in the absence of growth factors (Ctrl) or the presence of TGF-β1 (T), BMP-12 (B), or CTGF (C), and all resulting combinations (TB, TC, BC, and TBC). Gene expression levels are presented relative to that of BMSCs (BMSC-P2). As a single factor, TGF-β1 led to the greatest upregulation of expression of all tenogenic genes (Scx, Col1, Thbs4, TnC, Egr1, and Tnmd). Expression of all tenogenic marker genes was upregulated in all the combination groups where TGF-β1 was present. Data are presented as mean ± SD. All groups not connected by a common letter are significantly different (p < .05). Abbreviations: BMP12, bone morphogenetic protein; BMSC, bone marrow-derived mesenchymal stem cell; CTGF, connective tissue growth factor; Ctrl, control; TGFβ1, transforming growth factor β1.
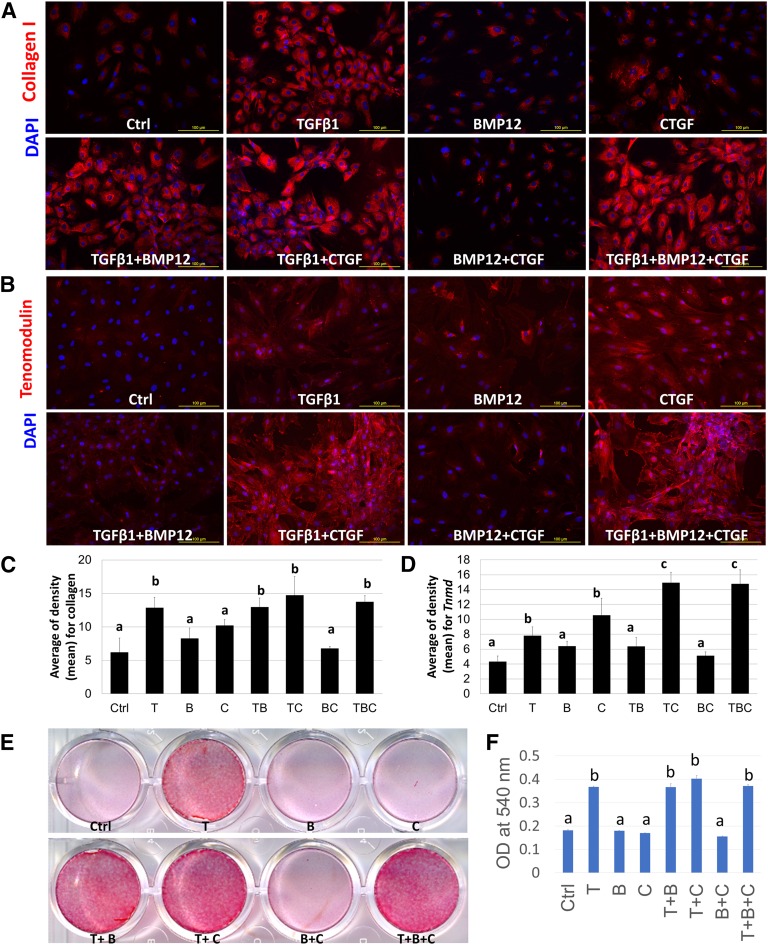
Differentiation toward the tenogenic lineage was characterized by immunofluorescence staining for tendon matrix protein collagen type I and tendon-specific marker tenomodulin. The results showed that TGF-β1 alone and with other growth factors significantly promoted collagen type I production compared with the control and other groups (Fig. 3A, 3C), which is consistent with mRNA expression results. Additionally, immunofluorescence staining of tenomodulin showed higher levels detected in the TGF-β1/CTGF and TGF-β1/BMP12/CTGF combination treatment groups (Fig. 3B). Quantification of immunofluorescence staining showed that the TGF-β1/CTGF and TGF-β1/BMP12/CTGF groups showed the highest levels of both collagen type I and tenomodulin expression (Fig. 3C, 3D), whereas no significant difference was detected between the two groups. Furthermore, Sirius red staining was conducted to evaluate the extracellular matrix, especially collagen secretion and production, which is the major component in tendon tissue. The intensity of Sirius red staining was significantly higher with TGF-β1, alone and with other growth factors, than in groups without TGF-β1 (Fig. 3E). Quantification data confirmed that TGF-β1 is the most potent factor in promoting collagen production; no synergetic effect was observed with other growth factors (Fig. 3F).
Figure 3.
Expression of collagen and tenomodulin in BMSCs induced with various growth factor treatments. Immunofluorescence staining of collagen type I (A) and tenomodulin (B) at day 7 was significantly upregulated in the groups treated with TGF-β1 and CTGF. Scale bars = 100 µm. Quantification data of immunofluorescence staining of collagen type I (C) and tenomodulin (D) was present. (E): Sirius red staining for collagen deposition evaluation in BMSCs cultured in the absence of growth factors (Ctrl) or the presence of TGF-β1 (T), BMP-12 (B), or CTGF (C) and all resulting combinations at day 7, showing the increased collagen production in the groups of T, T+B, T+C, and T+B+C. (F): Quantification data of Sirius red staining. Data are presented as mean ± SD. All groups not connected by a common letter are significantly different (p < .05). Abbreviations: BMP12, bone morphogenetic protein; BMSC, bone marrow-derived mesenchymal stem cell; CTGF, connective tissue growth factor; Ctrl, control; TGFβ1, transforming growth factor β1; DPAI, 4′,6-diamidino-2-phenylindole; OD, optical density.
Stepwise Tenogenic Differentiation Strategy for BMSCs
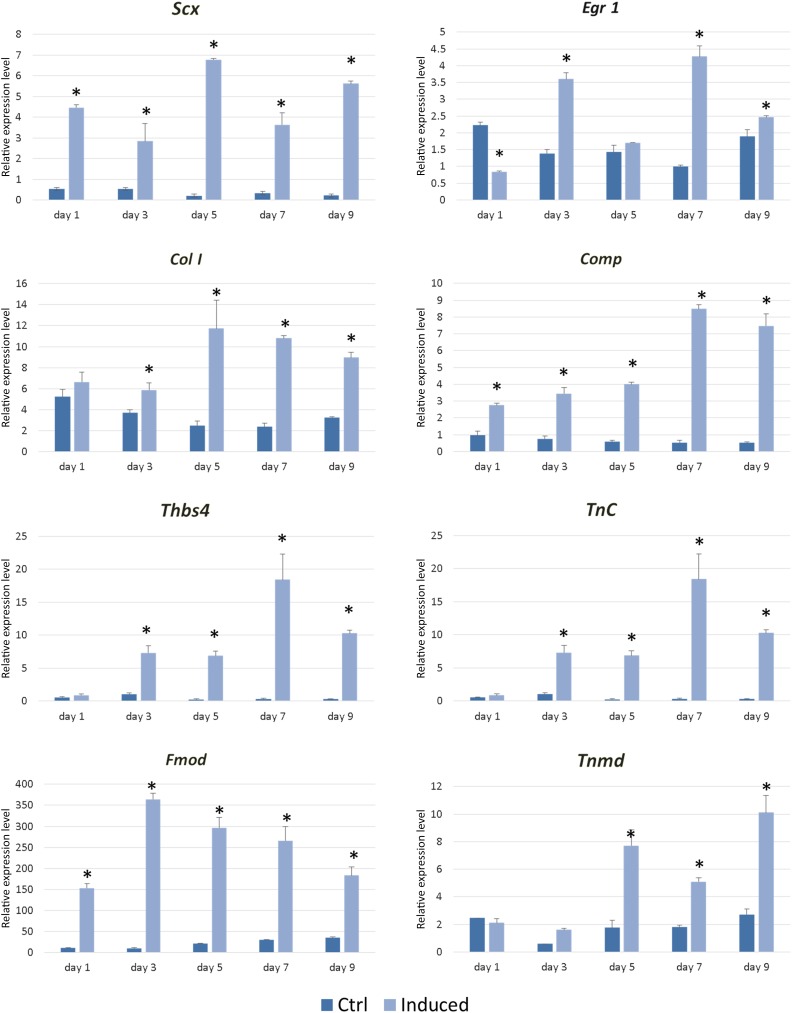
Based on these comparative studies of TGF-β1, BMP12, CTGF, and their combinations on tenogenic induction of BMSCs, it was found that TGF-β1 alone significantly and efficiently induced teno-lineage-specific gene scleraxis expression and collagen production. In addition, TGF-β1 combined with CTGF elevated tenomodulin mRNA and protein expression at day 7. Hence, the stepwise tenogenic differentiation approach was established by first using TGF-β1 stimulation for 3 days, followed by combination with CTGF for another 7 days. Gene expression analysis during this inductive process showed that this stepwise protocol initiated and maintained the high efficiency of tenogenic differentiation of BMSCs, as evidenced by significantly higher expression of Scx, Egr1, Col I, Comp, Tnc, Thbs4, Fmod, and Tnmd (Fig. 4). The efficacy of this stepwise tenogenic differentiation approach for BMSCs was further evaluated in a model of in vivo tendon repair and regeneration.
Figure 4.
Teno-lineage marker gene expression in bone marrow-derived stem cells during the stepwise tenogenic differentiation approach by first using transforming growth factor β1 stimulation for 3 days, followed by combination with connective tissue growth factor for another 7 days. The expression of Scx, Egr1, Col I, Comp, Tnc, Thbs4, Fmod, and Tnmd is presented at days 1, 3, 5, 7, and 9. ∗, p < .05.
In Vivo Ectopic Neotendon Formation
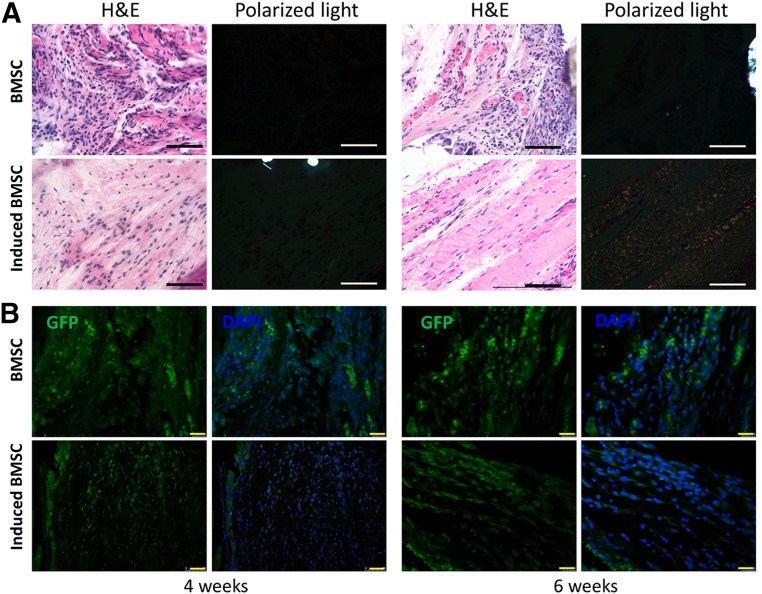
In the in vivo ectopic implantation model, engineered tendons by induced BMSCs using the stepwise tenogenic differentiation approach or uninduced BMSCs were implanted subcutaneously into SCID mice. We found that in the induced BMSC group, a greater number of cells exhibited spindle-shaped morphology and organized collagen deposition at both 4 and 6 weeks postimplantation (Fig. 5A), whereas the cells in the BMSC group showed round shapes in random arrangements (Fig. 5A). H&E staining showed that the matrices of the induced BMSCs were dense, whereas the BMSC groups were filled with loose, disarranged soft tissue (Fig. 5). Detection of GFP-tracked BMSCs showed that implanted BMSCs were present subcutaneously within the mice for at least 6 weeks and participated in ectopic new tissue regeneration in both groups (Fig. 5B). Sections were also observed under polarized light, and bands of collagen fibers were detected only in the induced BMSC group at 6 weeks postimplantation. This implied that the induced BMSCs could form tendon-like tissue in vivo and better arrangement of extracellular matrices.
Figure 5.
Ectopic neotendon formation by induced BMSCs. (A): H&E staining showing the histology of ectopic neotendon formation at 4 and 6 weeks postimplantation. Scale bars = 50 µm. Polarized light microscopy image showing the maturation of the newly formed collagen fibrils. Scale bars = 50 µm. (B): GFP fluorescence images showing implanted BMSC survival in neotissue site at 4 and 6 weeks postimplantation. Scale bars = 25 µm. Abbreviations: BMSC, bone marrow-derived stem cell; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; H&E, hematoxylin & eosin.
Induced BMSC Enhances Rat Patellar Tendon Repair
To further evaluate the tendon repair potential of the induced BMSCs in situ, a patellar tendon injury model was used.
Histology of the Repaired Tendon
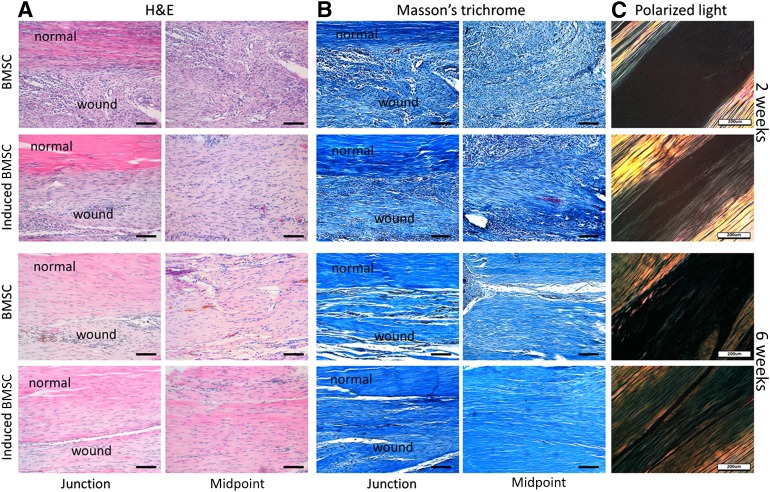
After 2 weeks, histology showed that more cells exhibited spindle-shaped morphology in the induced BMSC group (Fig. 6). Whereas the cell morphology in the BMSC group showed relatively round shapes (Fig. 6), Masson trichrome staining showed that the collagen fibrils in the induced BMSC group were aligned along the axis of tensile load (Fig. 6). In the BMSC group, the extracellular matrices in the repair site were relatively disorganized and had more immune cell infiltration and vascular components (Fig. 6).
Figure 6.
Histology result of repaired patella tendon at 2 and 6 weeks after healing. (A): H&E staining showing histology of the junction between normal tendon and neotendon, as well as the midpoint of neotendon formation at 2 and 6 weeks postimplantation. Scale bars = 100 µm. (B): Masson’s trichrome staining showing deposited collagen at the repaired tissue site at 2 and 6 weeks postimplantation. Scale bars = 100 µm. (C): Polarized light microscopy image showing maturation of the newly formed collagen fibrils. Scale bars = 200 µm. Abbreviations: BMSC, bone marrow-derived stem cell; DPAI, 4′,6-diamidino-2-phenylindole; H&E, hematoxylin & eosin.
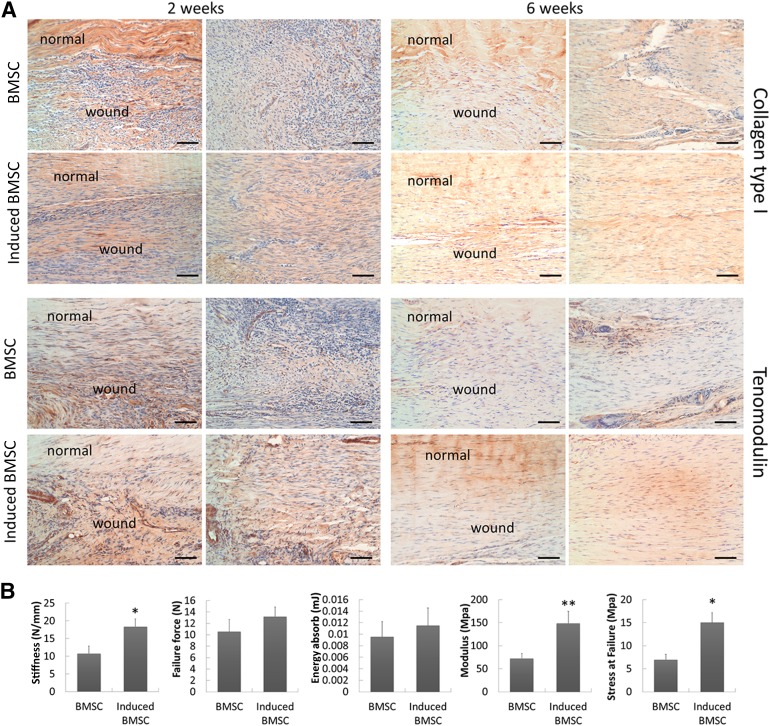
After 6 weeks, the histologic appearance of the neotendons in the induced BMSC group was more like native tendon structure, with fewer cell components and apparent bands of dense collagen fibers filling in the gap tissues (Fig. 6). There were denser collagen fibers and continuous tissues at the junction site formed in the induced BMSC group, as evidenced by Masson trichrome staining (Fig. 6). Collagen I immunohistochemistry staining was more intense in the induced BMSC group at both 2 and 6 weeks postimplantation, suggesting enhanced collagen type I production and arrangement (Fig. 7A). Tenomodulin immunohistochemistry staining revealed more positive cells in the induced BMSC group than in the BMSC group at junctions and neotendon formation sites (Fig. 7A). We also used polarized light microscopy to compare the collagen fiber maturation of the two groups. The induced BMSC group showed more mature and organized collagen fibers in the repaired site than the BMSC group at both 2 and 6 weeks postimplantation (Fig. 6).
Figure 7.
Immunostaining of tendon markers and mechanical testing results showed improved tendon healing quality in the group treated with induced BMSCs. (A): Immunohistochemistry of collagen I and tenomodulin expression of repaired tendon at 2 and 6 weeks. Scale bars = 100 µm. (B): Mechanical properties of repaired tendon at 6 weeks in induced BMSC and control groups. Data are presented as mean ± SD. n = 7. ∗, p < .05; ∗∗, p < .01. Abbreviation: BMSC, bone marrow-derived stem cell.
Mechanical Properties of the Repaired Tendons
To further correlate tissue structural features with their mechanical properties, harvested tendons (n = 7 for each group) were subjected to mechanical testing 6 weeks after surgery. The induced BMSC group had better mechanical properties than those of the controls. The stiffness (18.38 ± 2.19 N/mm vs. 10.78 ± 2.08 N/mm) was significantly higher in the induced BMSC group (Fig. 7B), which corresponded to 88% of the value obtained for intact rat tendon. The stress at failure in the induced BMSC treatment group was 2.15-fold that of the control group (15.04 ± 2.13 MPa vs. 6.98 ± 1.13 MPa, p < .05) (Fig. 7B), 63% and 29% of that of intact rat patellar tendon, respectively. The modulus in the induced BMSC group was twofold higher than that of the control group (148.75 ± 26.15 MPa vs. 72.47 ± 10.5 N, p < .05) (Fig. 7B), 84% and 41% of that of intact rat patellar tendon.
Discussion
The present study sought to investigate a reliable method to enhance tenogenic differentiation of BMSCs and generate functional neotendon tissue in vivo. First, three potential tenogenic factors, TGF-β1, BMP12, and CTGF, were chosen to evaluate their single or combined use on tenogenic differentiation of BMSCs. Notably, TGF-β1 was the most potent single factor that stimulates tenogenic gene upregulation and extracellular matrix production. BMSCs responded better to combined TGF-β1/CTGF and TGF-β1/BMP12/CTGF stimulation, as evidenced by the significant upregulation of tenomodulin and collagen type I protein expression. Thus, we used TGF-β1 to initiate tenogenesis in BMSCs by increasing transcription factor Scx significantly, followed by supplementation of TGF-β1/CTGF to induce and maintain full teno-lineage commitment. This stepwise tenogenic differentiation approach achieved optimal results within 10 days of induction. Finally, the induced BMSCs by this stepwise strategy had enhanced qualities for neotendon formation in both an ectopic tendon formation model and a rat patella tendon injury model.
Despite many studies using growth factor supplementation for tenogenic differentiation of BMSCs, with varying degrees of success, efficacy is hard to evaluate because of differences in cells, duration, and readout indices. Hence, it is essential to compare the potential growth factors shoulder-to-shoulder to conclude which is the most potent factor for tenogenic differentiation. Because adult tissue regeneration is generally thought to recapitulate developmental processes, insights from developmental biology have been considered important for tendon repair [23]. It was demonstrated that TGF-β and fibroblast growth factor (FGF) are the main growth factors involved in tendon development by embryologic experiments and genetic analyses [20, 24]. However, studies showed that there was no positive tenogenic effect of FGF4 on adult stem/progenitor cell differentiation [20, 25]. Application of human recombinant TGF-β ligands led to a significant increase in the mRNA expression level of tenogenic genes, whereas there was no significant difference between different ligands and no synergetic effect [18–20]. Thus, we chose one of the TGF-β ligands as a representative tenogenic factor to compare with other candidates. Additionally, BMP-12, BMP-13, and BMP-14 showed similar effects on inducing MSC differentiation, such as enhanced extracellular matrix and tenogenic marker gene upregulation. CTGF has also been demonstrated to sufficiently promote MSCs and tendon stem/progenitor cells (TSPCs) to differentiate into teno-lineage both in vitro and in vivo. In this study, TGF-β1 single stimulus led to the strongest induction of the tendon-specific genes Scx, Bgn, Col11, Thbs4, Fmod, Tnc, and Fn (p < .01). Among them, Scx, Fmod, and TnC were particularly highly upregulated, to >20-fold. Moreover, the promoting effect of TGF-β1 on tenogenic gene expression was detected as early as day 3, and neither of the other two growth factors exhibited any effect at this time point; previous reports showed that the effect of CTGF on tenogenesis was generally achieved as long as day 14 [12, 26]. Taken together, these data indicate that TGF-β1 plays a more profound role in tenogenic differentiation than BMP12 and CTGF in mesenchymal stem cells.
Besides the induction of specific differentiation, the maintenance of teno-lineage phenotype deserves more attention and consideration. Maeda et al. [27] examined 11 candidate cytokines/growth factors for their role in maintaining Scx expression in adult tenocytes in vitro, including TGF-β1, -2, and -3; GDF5, 7, and 4; insulin-like growth factor 1; platelet-derived growth factor; epidermal growth factor; vascular endothelial growth factor; and BMP2. The results indicated that the addition of TGF-β1, -2, and -3 and GDF8 results in the retention of Scx-GFP expression [27]. This is consistent with our findings that TGF-β1 not only induced Scx expression but also maintain its expression for at least 10 days. However, it was reported that the expression of Egr1 and Tnmd was not induced after TGF-β2 exposure in a mouse mesenchymal stem cell line [28], which was also true in our study. Furthermore, TGF-β1/CTGF and TGF-β1/BMP12/CTGF combination treatment groups showed significant increases in Egr1 and Tnmd expression. For cost considerations, we used TGF-β1/CTGF combination treatment to induce maturation of BMSCs toward the teno-lineage, after TGF-β1 treatment.
How MSCs differentiate toward the tenogenic pathway is poorly understood. TGF-β signaling is critical for tendon development, as most of the tendons and ligaments in the limbs, trunk, tail, and head are lost in Tgfb2−/− and Tgfb3−/− double-mutant embryos [24]. Our recent study also demonstrated that Mkx induces the expression of Scx, a transcription factor specific for tenocytes and their progenitors, via the TGF-β signaling pathway [18]. Moreover, it was reported that mechanical forces maintain the expression of Scx also through TGF-β/Smad2/3-mediated signaling [27], Therefore, the TGF-β signaling pathway is essential for tendon differentiation through Smad2/3 activation. However, TGF-β is also essential for chondrogenesis and osteogenesis. Under the conditions used in the study, tendon differentiation is dominant over chondrogenesis. The expression of Sox9 increased at day 3 but decreased gradually during the optimized stepwise tenogenic induction protocol. However, the mature chondrocyte marker collagen II was undetectable in BMSC culture in the absence (Ctrl) or presence of exogenous factors at both day 3 and day 7. These results indicated that successful chondrogenesis also requires 3D culture conditions, a combination of other factors (such as insulin/transferrin/sodium selenite, bovine serum albumin, and dexamethasone in chondrogenic media), or longer induction time.
Hurle and colleagues reported that TGF-β coordinates cartilage and tendon differentiation, which is mediated by stage-dependent regulation of transcriptional signaling repressors [29, 30]. The expression of CTGF has been shown to be involved in tendon healing processes and has been shown to favor fibrogenesis in connective tissue healing over ectopic mineralization [14]. Therefore, CTGF may help stem cells to follow the tenogenesis pathway rather than osteogenesis. A recent report suggested that CTGF-induced tenogenic differentiation was regulated by focal adhesion kinase and extracellular signal-regulated kinase 1/2 signaling [26]. The incomplete description of how CTGF’s signaling pathway crosstalks with the TGF-β signaling pathway is a limitation of the present study that warrants future in-depth investigation to unravel the specific mechanisms by which TGF-β1 and CTGF regulate tenogenesis.
In addition to BMSCs, tendon stem/progenitor cells have been studied as seed cells for tendon repair. It was reported that tendon stem/progenitor cells not only expressed higher level of teno-lineage genes but also exhibited higher clonogenicity compared with BMSCs [31]. Further studies should try to evaluate the current stepwise tenogenic differentiation approach on tendon stem/progenitor cells. Another limitation of our study was that we did not purposely study the most optimal growth factor concentrations. We have used reported workable concentrations based on single-stimulus tenogenic conditions in the current study; optimizing growth factor stimulation duration and concentration shall be the subject of future studies.
Conclusion
The present study focused on approaches using growth factors for tenogenesis, and the key tenogenic growth factors reported were compared with tenogenic differentiation by induced BMSCs. We established a stepwise inductive approach to induce tenogenesis of BMSCs by using TGF-β1 and TGF-β1/CTGF sequentially, which are efficient and effective for induction of tenogenic differentiation both in vitro and in vivo. Moreover, we found that the application of this stepwise tenogenic differentiation approach on BMSCs before transplantation promoted the functional repair of injured tendons, in contrast to BMSCs without induction. These findings provide important information for future tendon tissue engineering applications. The reliable BMSC tenogenic differentiation approach not only serves as a platform for further underlying molecular mechanisms studies, it also can be used to enhance cell therapy outcome in treating tendon disorders.
Supplementary Material
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation of China (LR14H060001), NSFC grants (81522029, 81401781), and the Key Scientific and Technological Innovation Team of Zhejiang Province (2013TD11). This study was supported in part by the SMART program, Lui Che Woo Institute of Innovative Medicine, Faculty of Medicine, The Chinese University of Hong Kong. This research project was made possible by resources donated by Lui Che Woo Foundation Limited. Y.Z. was a recipient of a postdoctoral research fellowship from the Faculty of Medicine, The Chinese University of Hong Kong (June 2014 to June 2015).
Author Contributions
Z.Y.: manuscript writing, collection and/or assembly of data, data analysis and interpretation; J.G., T.-y.W., L.-l.X., S.-e.L., and Y.-x.S.: collection and/or assembly of data; X.C.: collection and/or assembly of data, data analysis and interpretation; K.-M.C.: conception and design, financial support; H.O.: final approval of manuscript; G.L.: conception and design, financial support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
G.L. has uncompensated intellectual property rights and is an uncompensated consultant. The other authors indicated no potential conflicts of interest.
References
- 1.Aslan H, Kimelman-Bleich N, Pelled G, et al. Molecular targets for tendon neoformation. J Clin Invest. 2008;118:439–444. doi: 10.1172/JCI33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835–841. doi: 10.1177/0363546509359679. [DOI] [PubMed] [Google Scholar]
- 3.Maletius W, Gillquist J. Long-term results of anterior cruciate ligament reconstruction with a Dacron prosthesis. The frequency of osteoarthritis after seven to eleven years. Am J Sports Med. 1997;25:288–293. doi: 10.1177/036354659702500303. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF, Tullius R, Kokini K, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977–985. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 5.Milthorpe BK. Xenografts for tendon and ligament repair. Biomaterials. 1994;15:745–752. doi: 10.1016/0142-9612(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 6.Lui PP, Cheuk YC, Lee YW, et al. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J Orthop Res. 2012;30:37–46. doi: 10.1002/jor.21495. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Yin Z, Chen JL, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012;2:977. doi: 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Yin Z, Chen JL, et al. Scleraxis-overexpressed human embryonic stem cell-derived mesenchymal stem cells for tendon tissue engineering with knitted silk-collagen scaffold. Tissue Eng Part A. 2014;20:1583–1592. doi: 10.1089/ten.TEA.2012.0656. [DOI] [PubMed] [Google Scholar]
- 9.Yin Z, Chen X, Zhu T, et al. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9:9317–9329. doi: 10.1016/j.actbio.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Yin Z, Chen X, Song HX, et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials. 2015;44:173–185. doi: 10.1016/j.biomaterials.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Yin Z, Chen X, Chen JL, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 12.Ni M, Rui YF, Tan Q, et al. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials. 2013;34:2024–2037. doi: 10.1016/j.biomaterials.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Yuda A, Maeda H, Fujii S, et al. Effect of CTGF/CCN2 on osteo/cementoblastic and fibroblastic differentiation of a human periodontal ligament stem/progenitor cell line. J Cell Physiol. 2015;230:150–159. doi: 10.1002/jcp.24693. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Shah B, Moioli EK, et al. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berasi SP, Varadarajan U, Archambault J, et al. Divergent activities of osteogenic BMP2, and tenogenic BMP12 and BMP13 independent of receptor binding affinities. Growth Factors. 2011;29:128–139. doi: 10.3109/08977194.2011.593178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen H, Gelberman RH, Silva MJ, et al. BMP12 induces tenogenic differentiation of adipose-derived stromal cells. PLoS One. 2013;8:e77613. doi: 10.1371/journal.pone.0077613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Zhang C, Zhu S, et al. Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells. 2015;33:443–455. doi: 10.1002/stem.1866. [DOI] [PubMed] [Google Scholar]
- 19.Hagerty P, Lee A, Calve S, et al. The effect of growth factors on both collagen synthesis and tensile strength of engineered human ligaments. Biomaterials. 2012;33:6355–6361. doi: 10.1016/j.biomaterials.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Havis E, Bonnin MA, Olivera-Martinez I, et al. Transcriptomic analysis of mouse limb tendon cells during development. Development. 2014;141:3683–3696. doi: 10.1242/dev.108654. [DOI] [PubMed] [Google Scholar]
- 21.Meng F, Rui Y, Xu L, et al. Aqp1 enhances migration of bone marrow mesenchymal stem cells through regulation of FAK and β-catenin. Stem Cells Dev. 2014;23:66–75. doi: 10.1089/scd.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodship AE, Birch HL. Cross sectional area measurement of tendon and ligament in vitro: A simple, rapid, non-destructive technique. J Biomech. 2005;38:605–608. doi: 10.1016/j.jbiomech.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Nourissat G, Berenbaum F, Duprez D. Tendon injury: From biology to tendon repair. Nat Rev Rheumatol. 2015;11:223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 24.Pryce BA, Watson SS, Murchison ND, et al. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech. 2014;47:214–222. doi: 10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CH, Lee FY, Tarafder S, et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 2015;125:2690–2701. doi: 10.1172/JCI81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda T, Sakabe T, Sunaga A, et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564–3576. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorda-Diez CI, Montero JA, Martinez-Cue C, et al. Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J Biol Chem. 2009;284:29988–29996. doi: 10.1074/jbc.M109.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorda-Diez CI, Montero JA, Choe S, et al. Ligand- and stage-dependent divergent functions of BMP signaling in the differentiation of embryonic skeletogenic progenitors in vitro. J Bone Miner Res. 2014;29:735–748. doi: 10.1002/jbmr.2077. [DOI] [PubMed] [Google Scholar]
- 31.Tan Q, Lui PP, Rui Y, et al. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18:840–851. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.