This study demonstrates that generation of marrow-containing ossicles through a cartilage intermediate has relevance to develop human organotypic models for bone or hematopoietic cells and to engineer grafts for bone regeneration. Results show that despite their debated skeletal progenitor nature, human adipose-derived stromal cells can generate bone organs through endochondral ossification when suitably primed in vitro.
Keywords: Adipose-derived stromal cells, Differentiation, Endochondral ossification, Bone organ, Tissue engineering
Abstract
Recapitulation of endochondral ossification (ECO) (i.e., generation of marrow-containing ossicles through a cartilage intermediate) has relevance to develop human organotypic models for bone or hematopoietic cells and to engineer grafts for bone regeneration. Unlike bone marrow-derived stromal cells (also known as bone marrow-derived mesenchymal stromal/stem cells), adipose-derived stromal cells (ASC) have so far failed to form a bone organ by ECO. The goal of the present study was to assess whether priming human ASC to a defined stage of chondrogenesis in vitro allows their autonomous ECO upon ectopic implantation. ASC were cultured either as micromass pellets or into collagen sponges in chondrogenic medium containing transforming growth factor-β3 and bone morphogenetic protein-6 for 4 weeks (early hypertrophic templates) or for two additional weeks in medium supplemented with β-glycerophosphate, l-thyroxin, and interleukin1-β to induce hypertrophic maturation (late hypertrophic templates). Constructs were implanted in vivo and analyzed after 8 weeks. In vitro, ASC deposited cartilaginous matrix positive for glycosaminoglycans, type II collagen, and Indian hedgehog. Hypertrophic maturation induced upregulation of type X collagen, bone sialoprotein, and matrix metalloproteinase13 (MMP13). In vivo, both early and late hypertrophic templates underwent cartilage remodeling, as assessed by MMP13- and tartrate-resistant acid phosphatase-positive staining, and developed bone ossicles, including bone marrow elements, although to variable degrees of efficiency. In situ hybridization for human-specific sequences and staining with a human specific anti-CD146 antibody demonstrated the direct contribution of ASC to bone and stromal tissue formation. In conclusion, despite their debated skeletal progenitor nature, human ASC can generate bone organs through ECO when suitably primed in vitro.
Significance
Recapitulation of endochondral ossification (ECO) (i.e., generation of marrow-containing ossicles through a cartilage intermediate) has relevance to develop human organotypic models for bone or hematopoietic cells and to engineer grafts for bone regeneration. This study demonstrated that expanded, human adult adipose-derived stromal cells can generate ectopic bone through ECO, as previously reported for bone marrow stromal cells. This system can be used as a model in a variety of settings for mimicking ECO during development, physiology, or pathology (e.g., to investigate the role of BMPs, their receptors, and signaling pathways). The findings have also translational relevance in the field of bone regeneration, which, despite several advances in the domains of materials and surgical techniques, still faces various limitations before being introduced in the routine clinical practice.
Introduction
In most vertebrates, bone development occurs through two different mechanisms. These are typically referred to as (a) intramembranous ossification (IMO), where a direct differentiation of mesenchymal progenitor cells into bone-forming osteoblasts takes place, and (b) endochondral ossification (ECO), where mesenchymal progenitor cells first generate a cartilaginous hypertrophic matrix, which provides the template for remodeling into bone tissue [1]. Although both processes are observed in postnatal pathophysiological conditions, ECO is the dominant mode of ossification for the natural healing of bone fractures [2].
Engineering cell-based systems that recapitulate ECO offers the opportunity to investigate processes of bone development and functional mechanisms of hematopoietic stem cell niches in organotypic models, with the unique feature of using human cells, possibly from adult individuals [3]. The same approach is also relevant to generate grafts with intrinsic bone-forming capacity to replace substantial bone losses or enhance bone healing in critical clinical settings [4, 5].
Human, bone marrow-derived stromal cells (BMSC, also known as bone marrow-derived mesenchymal stromal/stem cells) have been demonstrated by different groups to have the capacity to induce bone formation in vivo by ECO, if appropriately primed toward chondrogenesis in vitro [3, 6–8]. The resulting ossicles displayed features of bone organs, including a fully functional hematopoietic compartment [9]. Adipose tissue has been identified as an alternative and abundant source of stromal progenitors, typically referred to as adipose stromal cells (ASC) [10]. Human ASC display several distinct translational advantages over mesenchymal stromal cells (MSC) from other tissues, such as bone marrow or umbilical cord, and have been used in a rapidly growing number of clinical trials [11]. Moreover, their biological properties and osteoprogenitor nature are generally recognized to be distinct from those of BMSC [8]. Indeed, only few studies reported the capacity of human ASC to acquire an osteoblastic phenotype and directly contribute to bone formation in formal in vivo assays of osteogenicity [12, 13]. These works indicate that ASC can generate bone tissue by IMO if exposed—in addition to a mineralized scaffold, as for BMSC [14–16]—to osteogenic triggers.
In the present study we investigated whether human ASC, induced in vitro to generate hypertrophic cartilage templates at different stages of maturation, can form bone through an ECO process. We further addressed specific questions related to (a) the activation of canonical pathways critical in ECO (e.g., Indian hedgehog), (b) the direct contribution of implanted ASC to the formed tissue, and (c) the establishment of bone marrow elements, typically formed in parallel with cartilage remodeling during ECO.
Materials and Methods
ASC Isolation, In Vitro Culture, Phenotype Characterization, and In Vivo Implantation
Samples of human adipose tissue were collected either as liposuction or as excision material obtained during routine surgical procedures (n = 3 donors). Informed consent was obtained preoperatively and the protocol approved by the local ethical committee (Ethikkommission beider Basel [EKKB], Ref. 78/07). Stromal vascular fraction (SVF) cells were isolated after enzymatic digestion of adipose tissue and centrifugation as previously described [17]. SVF cells were finally suspended in complete medium (CM), consisting of α-minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 1% HEPES, 1% sodium pyruvate, and 1% penicillin–streptomycin–glutamin (PSG) solution and filtered through a 100-μm strainer (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com). Cells were seeded onto tissue culture plates at a density of 3 × 103 cells per cm2 for monolayer (two-dimensional) expansion and cultured in CM supplemented with 5 ng/ml fibroblast growth factor-2 (FGF-2) (R&D Systems, Minneapolis, MN, https://www.rndsystems.com) for 7–8 days until subconfluence. For phenotype characterization, ASC were resuspended into 200 μl of 0.5% bovine serum albumin in phosphate-buffered saline (PBS) (FACS buffer) with fluorochrome-conjugated antibodies against the indicated protein or an isotype control and were incubated for 30 minutes at 4°C. The antibodies used were CD146-PE, CD90-FITC, CD73-PE, CD31-FITC, CD34-APC, IgG1-FITC, CD105-PE, IgG1-PE, and IgG-APC (all from Becton Dickinson and Company, Franklin Lakes, NJ). All the antibodies were used at a dilution of 1:50. Cells were washed twice with FACS buffer, resuspended in PBS, and analyzed with a FACSCalibur flow cytometer (Becton Dickinson and Company). For chondrogenic differentiation, 5 × 105 expanded ASC were either centrifuged to create a micromass pellet or seeded onto a 4-mm in diameter, 1-mm thick type I collagen-based cylindrical scaffold (Ultrafoam, Davol, Warwick, RI, http://www.davol.com). Both construct types were cultured in serum-free CM in the presence of 10 ng/ml transforming growth factor-β3 (TGF-β3) (R&D Systems), 10−7 M dexamethasone, 0.01 mM ascorbic acid (both from Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com), and 10 ng/ml bone morphogenetic protein 6 (BMP-6; R&D Systems) for 4 weeks (chondro-inductive medium). Half of the constructs were further cultured for 2 additional weeks in serum-free CM supplemented with 0.01 M β-glycerolphosphate, 10−8 M dexamethasone, 50 mM l-thyroxin, and 50 pg/ml interleukin1-β (hypertrophic medium, all from Sigma-Aldrich). Constructs were either analyzed or implanted in subcutaneous pouches in 4- to 6-week-old CD1 nu/nu female nude mice (cantonal permission BS1797) for 8 weeks, as previously described [3].
Gene Expression Analysis
The gene expression levels of the following matrix and osteoblastic markers are as follows: collagen types I, II, and X; matrix metalloproteinase13 (MMP13, also known as collagenase 3); BMP-4 and -7; runt-related transcription factor 2 (runx 2); and bone sialoprotein (BSP); as well as the hedgehog pathway markers Indian hedgehog homolog (IHH), its receptor protein patched homolog-1 (PTCH1), its mediator of signal transduction glioma-associated oncogene homolog-1 (GLI1), and sonic hedgehog homolog (SHH), which were quantified as previously described [3].
Histological Staining and Immunohistochemistry
After in vitro culture or in vivo implantation, the constructs were fixed in 1.5% paraformaldehyde overnight, decalcified by 7% EDTA if necessary, and embedded in paraffin. The sections (5 μm thickness) were stained with Haematoxylin and Eosin (H&E) (Avantor Performance Materials, Center Valley, PA, https://www.avantormaterials.com/), Safranin-O (Fluka, Sigma-Aldrich Chemie GmbH, Buchs, Switzerland, http://www.sigmaaldrich.com), and Masson’s trichrome staining (RAL Diagnostics, Martillac, France, http://www.ral-diagnostics.fr/en/) or for tartrate-resistant acid phosphatase (TRAP) activity with the leukocyte acid phosphatase kit (Sigma-Aldrich). To characterize the intracellular proteins and the extracellular matrix, immunohistochemical staining was performed as previously described [3] by using antibodies against the following proteins: Collagen type II (MPBiomedicals, Santa Ana, CA, http://www.mpbio.com) and type X (AbCam, Cambridge, U.K., http://www.abcam.com/), BSP (AbCam), MMP13 (AbCam), and human CD146 (Novocastra; Leica Biosystems, Muttenz, Switzerland, http://www.leicabiosystems.com). After incubation with a biotinylated secondary antibody and subsequently with an ABC-alkaline phosphatase complex, the specific staining was revealed by using Fast Red (all reagents from Dako, Bollschweil, Germany, http://www.dako.com/). Matched IgG control antibodies were used as a negative control.
In Situ Hybridization
To detect repetitive human-specific Alu sequences, chromogenic in situ hybridization was performed on paraffin sections of the in vivo constructs as described in [18]. To improve the binding of the probe, 1 base of the sequence was changed from 5′-cgaggcgggtggatcatgaggt-3′, reverse 5′-ttttttgagacggagtctcgc-3′ to 5′-cgaggcgggtgcatcatgaggt-3′, reverse 5′-ttttttgagacggagtctcgc-3′. For IHH, nonradioactive RNA in situ hybridization analysis on paraffin sections (in vitro and in vivo constructs and human fetal bone) was performed as described in [19], except that the RNase digestion step during the posthybridization washes was omitted. The human antisense riboprobes were synthesized using plasmids obtained from ImaGenes (Berlin, Germany, http://www.imagenes-bio.de, human IHH: NM_002181.2) and confirmed not to cross-react with murine transcripts.
Microtomography
After fixation in formalin and storage in PBS, micro-computed tomography (micro-CT) data were acquired from 8-week in vivo constructs by using a phoenix nanotom m scanner (General Electric, Fairfield, CT, http://www.ge.com) with 0.5-mm aluminum filtered x-rays (applied voltage 70 kV; current 260 μA). Transmission images were acquired during a 360° scan rotation with an incremental rotation step size of 0.25°. Reconstruction was made using a modified Feldkamp algorithm at an isotropic voxel size of 2.5 μm. Threshold-based segmentation and 3D measurement analyses (bone mineral density and volume) were performed using ImageJ software [20] with the BoneJ [21] and 3D Shape [22] extensions. Three-dimensional rendering of the structures was performed using VGStudio MAX 2.2 software (Volume Graphics, Heidelberg, Germany, http://www.volumegraphics.com/en/).
Statistical Analysis
Data are presented as mean ± SE. The significance of differences was evaluated by using analysis of variance (ANOVA) followed by the Bonferroni test for every set of data on which multiple comparisons were performed. p < .05 was considered statistically significant.
Results
In Vitro Maturation of Engineered Hypertrophic Cartilage Tissues
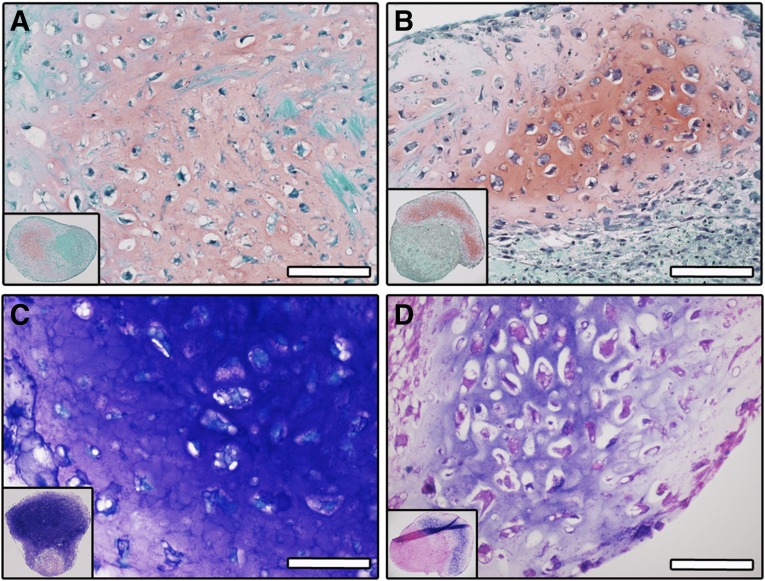
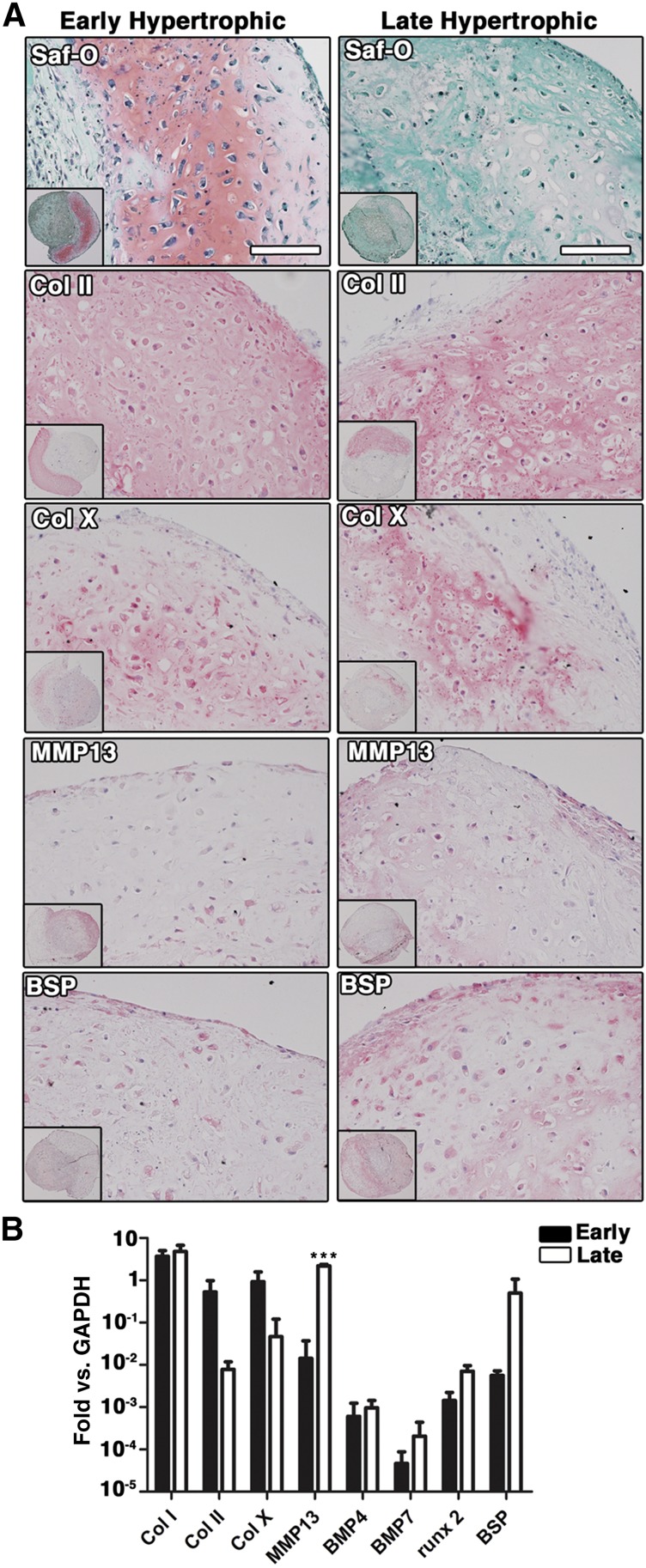
Before inducing differentiation, shortly expanded ASC were characterized for the expression of stromal and endothelial markers (n = 3 donors; supplemental online Fig. 1). ASC expressed CD105 (99.1% ± 0.5%), CD146 (32.8% ± 13.5%), CD73 (94.4% ± 0.9%), and CD90 (93.0% ± 5.0%). The presence of a small population of endothelial cells, double positive for CD34 and CD31, was also observed (1.9% ± 0.1%). To induce differentiation toward the chondrogenic lineage, ASC were cultured as micromass pellets or in type I collagen sponges (scaffolds), in the presence of chondro-inductive medium for 4 weeks. Formation of cartilaginous tissue was observed for constructs derived from all tested donors (n = 3 donors) in both experimental conditions (Fig. 1A, 1D) and at various degrees of efficiency (supplemental online Table 1, 9 of 27 for pellets and 2 of 2 for scaffolds, corresponding to 33% and 100% of the samples, respectively). A chondrocytic differentiation of ASC was evidenced by positive staining for glycosaminoglycans (GAG) by Safranin-O (Fig. 1A, 1B, for pellets and scaffolds, respectively) and by Toluidine Blue (Fig. 1C, 1D, for pellets and scaffolds, respectively) and by cell morphology, with large cells found in lacunae. The constructs contained not only cartilaginous tissue but also variable extents of fibrotic tissue, as evidenced by fast green counterstaining in the regions negative for Safranin-O. A certain degree of hypertrophy was already present in scaffold-based constructs after 4 weeks of culture, as indicated by the expression of collagen type X (Col X), MMP13, and BSP (Fig. 2A, left column). Because of the finding of limited and scattered expression of hypertrophy markers, these specimens were referred to as “early hypertrophic” constructs. To investigate the potential of the cartilaginous samples to further undergo hypertrophy, scaffold-based specimens were cultured for an additional 2 weeks in hypertrophic medium after the 4 weeks of chondrogenic medium. The 4- + 2-week scaffold-based constructs were compared with the 4-week ones for the expression of markers of hypertrophy. In the 4- + 2-week constructs, a strong reduction of GAG accumulation, together with moderately increased staining intensity for hypertrophy markers, including Col X, MMP13, and BSP, was observed (Fig. 2A, right column). Quantitative gene expression analysis showed a trend for downregulation of collagen type II (Col II) and Col X and an upregulation of MMP13, BMP4, BMP7, runx2, and BSP, following culture in hypertrophic medium, with only MMP13 being significantly increased (p < .001, Fig. 2B), suggesting more advanced hypertrophic traits. For this reason, the 4- + 2-week constructs were referred to as “late hypertrophic” constructs.
Figure 1.
In vitro generation of cartilaginous tissues. Shown are representative fields of Safranin-O and Toluidine Blue staining for glycosaminoglycans (GAG) of engineered pellets (A, C) and collagen-based scaffolds (B, D) after 4 weeks of in vitro culture in chondro-inductive medium. Scale bar = 50 µm.
Figure 2.
In vitro maturation of cartilaginous tissues. (A): Safranin-O staining and immunostaining for Col II, Col X, MMP13, and BSP of early (4 weeks, left column) and late (4 + 2 weeks) hypertrophic scaffold-based samples (right column). (B): Quantification (mean ± SD) of mRNA expression of chondrogenic and early and late hypertrophic markers in early (black bars) and late (white bars) hypertrophic scaffolds. ∗∗∗, p < .001 (two-way ANOVA with Bonferroni post-tests, n = 4 values per group). Scale bar = 100 µm. Abbreviations: BMP4, bone morphogenetic protein-4; BMP7, bone morphogenetic protein-7; BSP, bone sialoprotein; Col II, collagen type II; Col X, collagen type X; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP13, matrix metalloproteinase13; runx 2, runt-related transcription factor 2; Saf-O, Safranin-O.
Indian Hedgehog Is Upregulated During ASC Chondrogenic Differentiation In Vitro
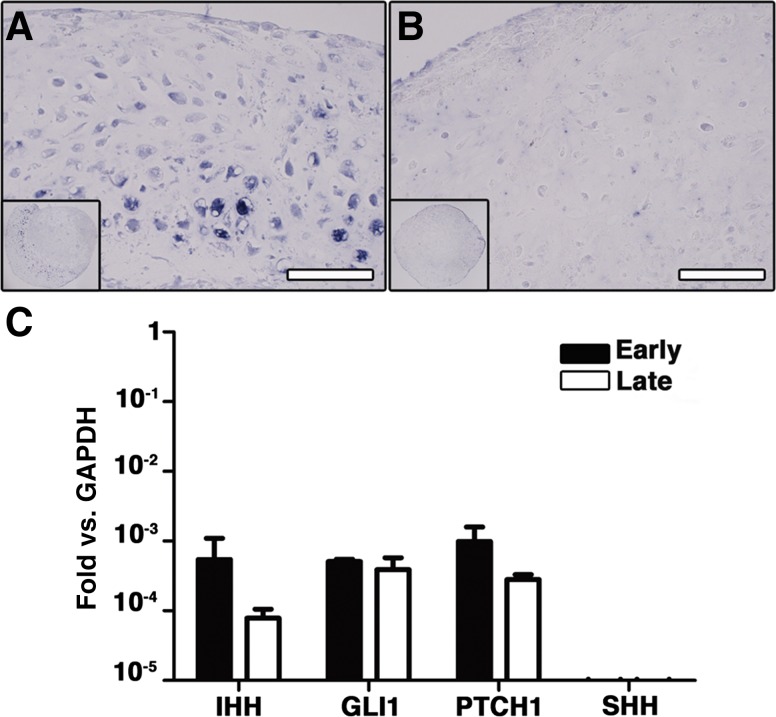
IHH is the central coordinator of endochondral bone growth and morphogenesis in vivo [23]. To investigate whether IHH and related genes were expressed in our system, in situ hybridization and quantification with quantitative RT-PCR were performed in early and late hypertrophic constructs. The IHH gene was expressed in the cartilaginous areas in early hypertrophic scaffold-based specimens (Fig. 3A). In the late hypertrophic constructs, instead, IHH was expressed only by scattered cells and with no specific pattern (Fig. 3B). Not only IHH, but also its receptor PTCH1, as well as GLI1, a mediator of the IHH signal transduction cascade, were expressed in both the early and late hypertrophic constructs (Fig. 3C), with no significant difference (p = N.S.) between the two conditions. In contrast, expression of sonic hedgehog (SHH), another regulator of limb outgrowth and closely related to IHH, was undetectable.
Figure 3.
In vitro activation of the IHH signaling pathway. Shown is in situ hybridization for IHH mRNA in early (4 weeks [A]) and late (4 + 2 weeks [B]) hypertrophic scaffold-based samples. (C): Quantification of mRNA expression (mean ± SD) for IHH, its receptor PTCH1, and GLI1 in early (white bar) and late (black bar) hypertrophic constructs. Scale bar = 50 µm. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLI1, glioma-associated oncogene homolog-1; IHH, Indian hedgehog; PTCH1, patched homolog-1; SHH, sonic hedgehog homolog.
ASC Form Bone Tissue In Vivo Through Endochondral Ossification
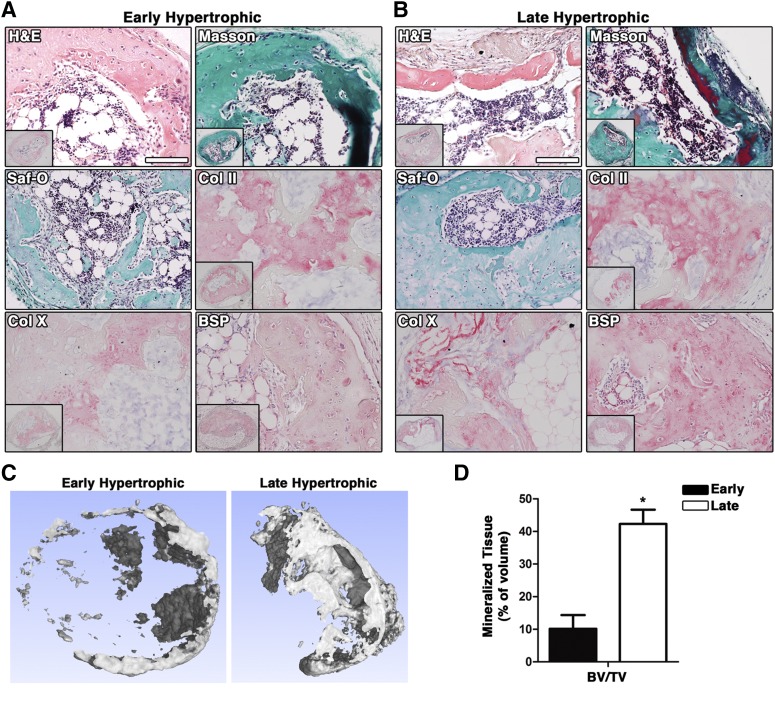
BMSC have been shown to undergo endochondral ossification in vivo starting from hypertrophic cartilaginous templates [3]. To investigate whether also cartilaginous constructs obtained with ASC could form bone through the endochondral pathway, early and late hypertrophic tissues were implanted subcutaneously into nude mice. For pellets, 10 of 35 for early and 24 of 34 for late hypertrophic constructs could be retrieved after in vivo implantation (supplemental online Table 1). For scaffold-based samples, instead, all the implanted constructs were recovered, regardless of whether hypertrophy was induced before in vivo implantation, indicating that they were more durable in vivo compared with pellets. In two donors of three, both early and late hypertrophic constructs showed strong remodeling and formation of bone tissue (supplemental online Table 1). Bone tissue was present in 12 of 34 recovered pellets from the three donors (3 of 10 for early and 9 of 24 for late hypertrophic, in 30% and 38% of the samples, respectively) and in 6 of 16 recovered scaffolds (3 of 8 for early and 3 of 8 for late hypertrophic, corresponding to 38% of the samples). When considering the number of implanted constructs, instead of the recovered ones only, 3 of 35 (9%) early and 9 of 34 (26%) late hypertrophic pellets generated bone tissue in vivo. Bone tissue formation was characterized by the presence of a dense collagen type I- and BSP-rich and glycosaminoglycan-negative matrix, as evidenced, respectively, by Masson trichrome and Safranin-O staining. The cells embedded inside this dense matrix resembled osteocytes, whereas cuboidal cells at the periphery of the matrix resembled lining osteoblasts and expressed BSP (representative images from scaffold-based constructs, Fig. 4A, 4B). The presence of remnants of hypertrophic cartilage, evidenced by positivity for Safranin-O, Col II, and Col X next to the newly formed ossicles, suggested that bone formation occurred through an endochondral ossification process (Fig. 4A, 4B). Different degrees of bone maturation were evidenced by the variable organization of collagen fibers/bundles under polarized light microscopy (supplemental online Fig. 2A for early hypertrophic scaffold-based constructs, and supplemental online Fig. 2B for late hypertrophic pellets) and by the scattered presence of elastic fibers, characterizing mature bone structures (Fig. 4, areas stained in dark red by Masson’s trichrome staining). In a few samples (supplemental online Table 1, 1 of 34 for implanted late pellets and 1 of 8 and 2 of 8 for early and late implanted scaffolds, respectively) the bone tissue formed was also associated with bone marrow (BM). In the scaffold-based constructs, quantitative analysis of micro-CT data (Fig. 4C, 4D) indicated that late hypertrophic constructs had 4-fold more mineralized tissue volume than early hypertrophic ones (p < .05). In early hypertrophic constructs, bone was mainly found as an outer shell, whereas in late hypertrophic constructs trabecular-like structures were also observed (Fig. 4B).
Figure 4.
Development of the cartilaginous tissues following ectopic in vivo implantation. Shown is characterization of early (4 weeks [A]) and late (4 + 2 weeks [B]) hypertrophic scaffold-based constructs after 8 weeks of in vivo implantation by histochemical (H&E and Masson’s trichrome staining), histological, and immunohistochemical analysis. (C): Tomographic reconstruction of early and late hypertrophic scaffold-based constructs after 8 weeks of implantation. (D): Quantification of mineralized tissue volume in early (black bar) and late (white bar) hypertrophic scaffold-based constructs. ∗, p < .05 (two-way ANOVA with Bonferroni post-tests, n = 3 values per group). Scale bar = 100 µm. Abbreviations: ANOVA, analysis of variance; BSP, bone sialoprotein; BV/TV, bone volume/total volume; Col II, collagen type II; Col X, collagen type X; H&E, hematoxylin and eosin; Saf-O, Safranin-O.
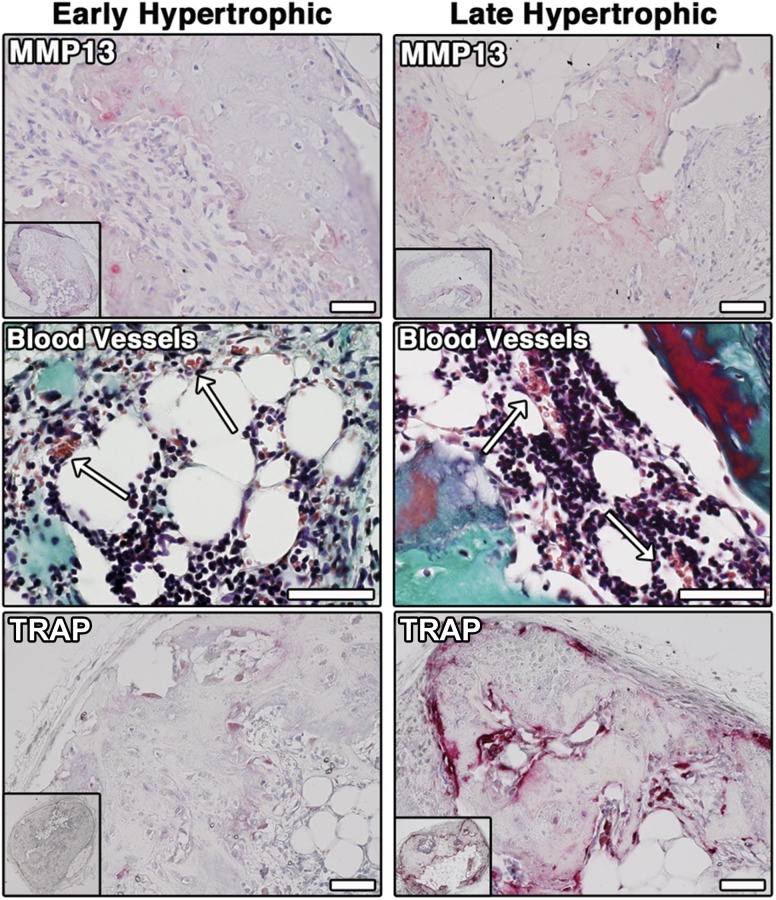
Characterization of Remodeling and Vascularization of Early and Late Hypertrophic Cartilage Tissues
The implanted, scaffold-based constructs were analyzed more in detail to characterize the in vivo remodeling and vascularization. MMP-13, which remodels the matrix to allow vascular ingrowth, was expressed in scattered regions both in early and in late hypertrophic constructs (Fig. 5, top). A matched IgG control antibody showed no staining (data not shown). Capillary and small blood vessels, containing erythrocytes (stained in brown by Masson’s trichrome staining), were found up to the center of the constructs and inside the BM (arrows; Fig. 5, middle). An intense TRAP activity revealed osteoclastic/chondroclastic remodeling at the rim of the newly formed bone in the late hypertrophic constructs (Fig. 5, bottom). TRAP activity was much less intense in early hypertrophic constructs.
Figure 5.
In vivo remodeling and vascularization. Immunohistochemical staining for MMP13 (top), Masson Trichrome staining for vascularization of the bone marrow cavity (white arrows, middle), and TRAP staining for osteoclasts/chondroclasts in early (4 weeks, bottom left) and late hypertrophic scaffold-based constructs (4 + 2 weeks, bottom right) are shown. Scale bar = 50 µm. Abbreviations: MMP13, matrix metalloproteinase13; TRAP, tartrate-resistant acid phosphatase.
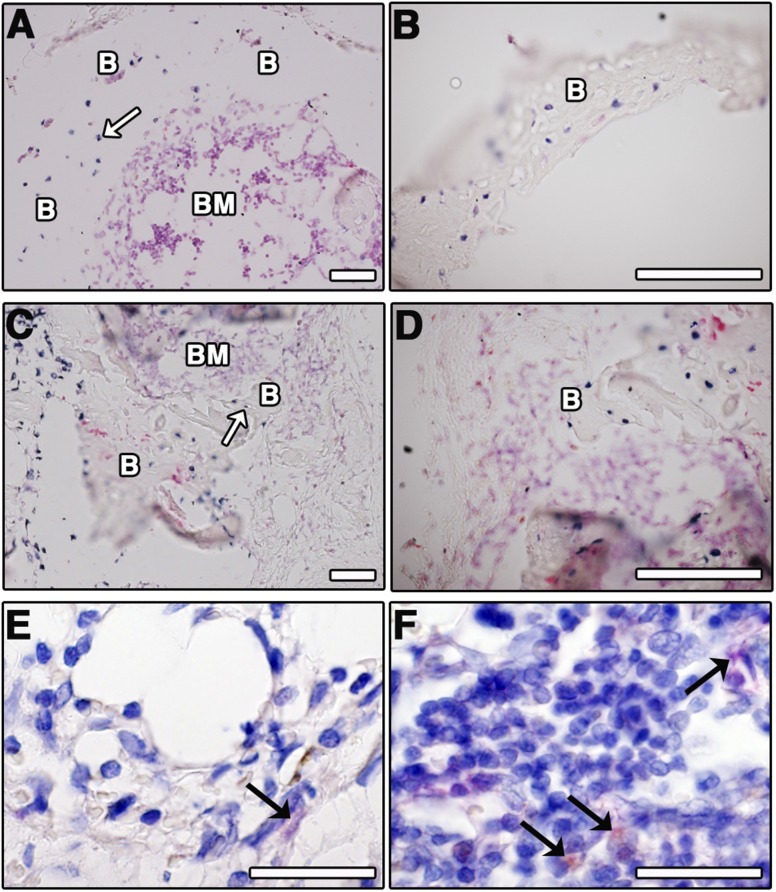
Implanted ASC Contribute to the Formation of Bone Tissue and BM Stroma
To investigate the direct contribution of implanted ASC in bone formation, histological sections of early and late hypertrophic scaffold-based constructs were stained by in situ hybridization for human Alu sequences. The presence of Alu-positive cells within the bone matrix was found both in early and in late hypertrophic constructs (white arrows; Fig. 6A, 6B and Fig. 6C, 6D, respectively). To determine whether implanted ASC contributed not only to bone formation, but also to the BM stroma, staining was performed with a human-specific antibody to CD146, a highly specific marker of stromal cells in the bone marrow [24]. In both early and late hypertrophic constructs, the presence of human CD146+ cells located within the BM stromal tissue was observed (black arrows; Fig. 6E, 6F, for early and late hypertrophic constructs, respectively). However, CD146+ cells were never found associated with blood vessels.
Figure 6.
Human cells contribute to the formation of bone tissue and bone marrow stroma. Shown is in situ hybridization for human specific Alu sequences (blue staining, white arrows) in early (4 weeks [A, B]) and late (4 + 2 weeks [C, D]) hypertrophic scaffold-based constructs. (E, F): Human origin CD146+ cells (black arrows) in the marrow stroma. Scale bar = 50 µm. Abbreviations: B, bone; BM, bone marrow.
Discussion
The present study demonstrates that expanded, human adult ASC can generate ectopic bone through ECO, as previously reported for BMSC [3, 6–8]. Several features of the biological processes involved in ECO by ASC and BMSC displayed strong analogies. For both cell types, hypertrophic chondrogenesis, assessed by the expression of Col X, MMP13, and BSP, was associated with the activation of IHH, the master gene regulating ECO during development [23], and its downstream targets GLI1 and PTCH1. Upon in vivo implantation, ASC-based hypertrophic templates induced MMP- and osteoclast-/chondroclast-mediated matrix remodeling as well as vascular ingrowth, ultimately leading to perichondral bone matrix apposition and the formation of bone ossicles, as observed for BMSC [9]. For both ASC and BMSC, a more mature stage of hypertrophy of the cartilaginous templates in vitro resulted in a larger amount of bone formation in vivo. Finally, bone tissues formed in mice by ECO from both cell sources included implanted human cells and contained bone marrow elements, embedding CD146-positive cells of human origin [25]. Whether those CD146-positive cells are arising from the CD146-positive expanded ASC used to generate the cartilaginous templates, as demonstrated for BMSC [24], and whether these two cell types differ in contributing to BM homing remain to be investigated.
The main difference in the ECO processes reproduced by ASC and BMSC was related to the initial chondrogenic induction. In fact, chondrogenesis by ASC in vitro required not only TGF-β, as typically sufficient for BMSC [26], but also an additional potent morphogen, namely BMP-6. This finding is in keeping with a previous study [27], although ASC from pediatric patients were reported to undergo chondrocytic differentiation with only TGF-β1 [28]. The activation of BMP signaling in cells from nonskeletal tissues, including fat, was recently suggested as a crucial condition to induce a switch into skeletal (and therefore also chondrogenic) progenitors [29]. Our data thus reinforce the concept of ASC as “inducible” but not “determined” progenitors, requiring reprogramming signals to acquire a skeletogenic capacity, at least under physiological conditions [29]. Interestingly, constitutive pathologic activation of BMP signaling through a mutation in the ACVR1/ALK2 gene leads to the onset of heterotopic ECO in the proximity of adipose tissue, as one of the hallmarks of fibrodisplasia ossificans progressiva (FOP) [30]. Moreover, it has been recently shown that also endothelial cells could differentiate toward the chondrogenic lineage if treated with TGF-β2 and BMP-4 [31]. Although our data show that the contamination of endothelial cells was minimal (less than 2%), a contribution by endothelial cells in the formation of the cartilaginous templates cannot be excluded.
Beyond the BMP requirement, also the repeatability of the ECO process by ASC—although not assessed in a direct comparative study—seemed to be lower than by BMSC. Interestingly, the observed bone formation frequency in scaffolds (30%) was similar to the frequency of chondrogenic induction reported here and in a previous study [32]. Although the efficiency of bone formation by early hypertrophic pellets was very low (9%), induction of hypertrophy increased both the rate of recovered implants and the amount of constructs containing bone tissue. In fact, late hypertrophic pellets showed similar yields of bone formation compared with scaffold-based samples (26% vs. 38% respectively). This suggests that the critical step in ECO by ASC is the initial priming to a hypertrophic cell phenotype and that when this is achieved, bone formation in vivo proceeds efficiently, as with BMSC. In this context, it is relevant to remark that the formation of a hypertrophic cartilaginous template by other cell sources is not always sufficient to initiate ECO in vivo. This was recently demonstrated with chondrogenically reprogrammed dermal fibroblasts by constitutive expression of Sox9, Klf4, and c-Myc [33], as well as with nasal chondrocytes [34]. Taken together, these results indicate that ASC, although requiring distinct conditions to enter chondrogenesis, which may be related to their epigenetic signature [8], have the ability to execute an ECO program, as opposed to phenotypically stable chondrocytes. One limitation of our study is related to the fact that pellet implants suffered from a limited rate of recovery and that scaffold constructs were based on cells from a single donor. Although our data are relevant toward outlining and proving in principle a biological process, further studies should include a larger number of samples generated using ASC from several donors to assess the reproducibility and robustness of the findings.
To the best of our knowledge, our study is the first to indicate the possibility for ASC to activate and directly contribute to an ECO process. Rabbit ASC engineered to overexpress BMP-2 were previously reported to induce ECO in a calvarial model, but the study could not conclude whether the process was merely induced by the delivery of the morphogen, as the role of implanted ASC was not assessed [35]. Human ASC were convincingly shown to participate in ectopic bone formation following chondrogenic preinduction in vitro [14], but no evidence of ECO was provided and bone tissue appeared to be formed by IMO. The apparent discrepancy of our results from those of the latter study could be related to the fact that here ASC were used after a limited in vitro expansion, without passaging, and implanted in the absence of a calcium phosphate-based carrier, which is known to prime stromal progenitors toward IMO [15].
The bone regenerative capacity of ASC through ECO now needs to be tested in orthotopic and possibly immunocompetent models of bone repair. To facilitate the translation of ASC-induced ECO into therapeutic applications, future studies could test the feasibility of inducing hypertrophic chondrogenesis directly in vivo. This could be achieved using freshly harvested cells from adipose tissue in an intraoperative setting, similar to what was recently demonstrated for ASC-induced IMO [36]. It will also be relevant to test whether the decellularized cartilaginous matrix produced by ASC will retain the property to instruct resident progenitors, as recently proposed for BMSC-generated hypertrophic templates [37] to develop an off-the-shelf material.
Conclusion
Adult human adipose-derived stromal progenitors are able to undergo a program of ECO, resembling developmental processes, if primed into a chondrogenic intermediate by stimulation with bone morphogenetic protein-6. This system can be used as a model in a variety of settings mimicking ECO during development, physiology, or pathology (e.g., to investigate the role of BMPs, their receptors, and signaling pathways). The findings have also translational relevance in the field of cell-based bone regeneration, which, despite several advances in the domains of materials and surgical techniques, still faces various limitations before being introduced in the routine clinical practice [38].
Supplementary Material
Acknowledgments
We are very grateful to Dr. Karoliina Pelttari (Basel, Switzerland) for scientific advice on the project. This study was supported by the Swiss National Science Foundation, SNF Grant 310030-138519 (to A.S. and I.M.).
Author Contributions
R.O.: provision of study material and patients, performing experiments, collection and assembly of data, data analysis and interpretation, manuscript writing; N.D.M.: conception and design, performing experiments, collection and assembly of data, data analysis, and interpretation, manuscript writing, final approval of manuscript; A.T.: conception and design, data collection, analysis, and interpretation; N.A.: provision of study material, collection and assembly of data; A.B.: conception and design, analysis and interpretation; F.L.: assembly and analysis of data; D.J.S.: financial and administrative support, provision of study patients; I.M.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript; A.S.: conception and design, financial and administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis PV, Kanakaris NK, Tsiridis E. Principles of internal fixation and selection of implants for periprosthetic femoral fractures. Injury. 2007;38:669–687. doi: 10.1016/j.injury.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadimitropoulos A, Scotti C, Bourgine P, et al. Engineered decellularized matrices to instruct bone regeneration processes. Bone. 2015;70:66–72. doi: 10.1016/j.bone.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res. 2014;100(suppl):S107–S112. doi: 10.1016/j.otsr.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Farrell E, Both SK, Odörfer KI, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janicki P, Kasten P, Kleinschmidt K, et al. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: Enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater. 2010;6:3292–3301. doi: 10.1016/j.actbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Reinisch A, Etchart N, Thomas D, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249–260. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scotti C, Piccinini E, Takizawa H, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordberg RC, Loboa EG. Our fat future: Translating adipose stem cell therapy. Stem Cells Transl Med. 2015;4:974–979. doi: 10.5966/sctm.2015-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon O, Song SJ, Yang HS, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun. 2008;369:774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 13.Scherberich A, Galli R, Jaquiery C, et al. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823–1829. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 14.Brocher J, Janicki P, Voltz P, et al. Inferior ectopic bone formation of mesenchymal stromal cells from adipose tissue compared to bone marrow: Rescue by chondrogenic pre-induction. Stem Cell Res. 2013;11:1393–1406. doi: 10.1016/j.scr.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Hattori H, Masuoka K, Sato M, et al. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J Biomed Mater Res B Appl Biomater. 2006;76:230–239. doi: 10.1002/jbm.b.30357. [DOI] [PubMed] [Google Scholar]
- 16.Hattori H, Sato M, Masuoka K, et al. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs. 2004;178:2–12. doi: 10.1159/000081088. [DOI] [PubMed] [Google Scholar]
- 17.Güven S, Mehrkens A, Saxer F, et al. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials. 2011;32:5801–5809. doi: 10.1016/j.biomaterials.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 18.Kasten P, Vogel J, Luginbühl R, et al. Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials. 2005;26:5879–5889. doi: 10.1016/j.biomaterials.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Chotteau-Lelièvre A, Dollé P, Gofflot F. Expression analysis of murine genes using in situ hybridization with radioactive and nonradioactively labeled RNA probes. Methods Mol Biol. 2006;326:61–87. doi: 10.1385/1-59745-007-3:61. [DOI] [PubMed] [Google Scholar]
- 20.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doube M, Kłosowski MM, Arganda-Carreras I, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–1079. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheets KG, Jun B, Zhou Y, et al. Microglial ramification and redistribution concomitant with the attenuation of choroidal neovascularization by neuroprotectin D1. Mol Vis. 2013;19:1747–1759. [PMC free article] [PubMed] [Google Scholar]
- 23.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 24.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Serafini M, Sacchetti B, Pievani A, et al. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 2014;12:659–672. doi: 10.1016/j.scr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Dickhut A, Pelttari K, Janicki P, et al. Calcification or dedifferentiation: Requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 27.Hennig T, Lorenz H, Thiel A, et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 28.Guasti L, Prasongchean W, Kleftouris G, et al. High plasticity of pediatric adipose tissue-derived stem cells: Too much for selective skeletogenic differentiation? Stem Cells Transl Med. 2012;1:384–395. doi: 10.5966/sctm.2012-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassem M, Bianco P. Skeletal stem cells in space and time. Cell. 2015;160:17–19. doi: 10.1016/j.cell.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medici D, Shore EM, Lounev VY, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osinga R, Menzi NR, Tchang LA, et al. Effects of intersyringe processing on adipose tissue and its cellular components: Implications in autologous fat grafting. Plast Reconstr Surg. 2015;135:1618–1628. doi: 10.1097/PRS.0000000000001288. [DOI] [PubMed] [Google Scholar]
- 33.Tam WL, O DF, Hiramatsu K, et al. Sox9 reprogrammed dermal fibroblasts undergo hypertrophic differentiation in vitro and trigger endochondral ossification in vivo. Cell Reprogram. 2014;16:29–39. doi: 10.1089/cell.2013.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pippenger BE, Ventura M, Pelttari K, et al. Bone-forming capacity of adult human nasal chondrocytes. J Cell Mol Med. 2015;19:1390–1399. doi: 10.1111/jcmm.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin WH, Tsai WB. In situ UV-crosslinking gelatin electrospun fibers for tissue engineering applications. Biofabrication. 2013;5:035008. doi: 10.1088/1758-5082/5/3/035008. [DOI] [PubMed] [Google Scholar]
- 36.Mehrkens A, Saxer F, Güven S, et al. Intraoperative engineering of osteogenic grafts combining freshly harvested, human adipose-derived cells and physiological doses of bone morphogenetic protein-2. Eur Cell Mater. 2012;24:308–319. doi: 10.22203/ecm.v024a22. [DOI] [PubMed] [Google Scholar]
- 37.Bourgine PE, Scotti C, Pigeot S, et al. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc Natl Acad Sci USA. 2014;111:17426–17431. doi: 10.1073/pnas.1411975111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grayson WL, Bunnell BA, Martin E, et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11:140–150. doi: 10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.