Many patients with chronic myocardial ischemia are not suitable for surgery and have no effective drug treatment. This study finds that umbilical cord-derived mesenchymal stromal cells transplanted by intracoronary delivery combined with two intravenous administrations was safe and could significantly improve left ventricular function, perfusion, and remodeling in a large-animal model of chronic myocardial ischemia.
Keywords: Umbilical cord, Mesenchymal stromal cells, Chronic myocardial ischemia, Ischemic heart disease, Ventricular remodeling
Abstract
Stem cell therapy has emerged as a new strategy for treatment of ischemic heart disease. Although umbilical cord-derived mesenchymal stromal cells (UC-MSCs) have been used preferentially in the acute ischemia model, data for the chronic ischemia model are lacking. In this study, we investigated the effect of UC-MSCs originated from Wharton’s jelly in the treatment of chronic myocardial ischemia in a porcine model induced by ameroid constrictor. Four weeks after ameroid constrictor placement, the surviving animals were divided randomly into two groups to undergo saline injection (n = 6) or UC-MSC transplantation (n = 6) through the left main coronary artery. Two additional intravenous administrations of UC-MSCs were performed in the following 2 weeks to enhance therapeutic effect. Cardiac function and perfusion were examined just before and at 4 weeks after intracoronary transplantation. The results showed that pigs with UC-MSC transplantation exhibited significantly greater left ventricular ejection fraction compared with control animals (61.3% ± 1.3% vs. 50.3% ± 2.0%, p < .05). The systolic thickening fraction in the infarcted left ventricular wall was also improved (41.2% ± 3.3% vs. 46.2% ± 2.3%, p < .01). Additionally, the administration of UC-MSCs promoted collateral development and myocardial perfusion. The indices of fibrosis and apoptosis were also significantly reduced. Immunofluorescence staining showed clusters of CM-DiI-labeled cells in the border zone, some of which expressed von Willebrand factor. These results suggest that UC-MSC treatment improves left ventricular function, perfusion, and remodeling in a porcine model with chronic myocardial ischemia.
Significance
Ischemic heart disease is the leading cause of death worldwide. Many patients with chronic myocardial ischemia are not suitable for surgery and have no effective drug treatment; they are called “no-option” patients. This study finds that umbilical cord-derived mesenchymal stromal cells transplanted by intracoronary delivery combined with two intravenous administrations was safe and could significantly improve left ventricular function, perfusion, and remodeling in a large-animal model of chronic myocardial ischemia, which provides a new choice for the no-option patients. In addition, this study used clinical-grade mesenchymal stem cells with delivery and assessment methods commonly used clinically to facilitate further clinical transformation.
Introduction
Despite the equipment and techniques that have been developed for percutaneous coronary intervention and coronary artery bypass grafting, many patients with chronic ischemic heart diseases (CHDs) cannot benefit from cardiac intervention or surgical operation because of severe or diffused occlusion of coronary arteries [1]. In recent years, many experimental [2, 3] and clinical findings [4] have suggested the therapeutic potential of stem cell therapy in ischemic heart disease, which provides a new option for these patients. However, most of these studies focus on acute ischemic heart disease. There are limited data in stem cell treatment in CHDs.
Among the numerous cell types used in cell therapy, mesenchymal stem cells (MSCs) is the most hopeful candidate to be translated from bench to bedside [5]. Although several preclinical and clinical studies using bone marrow-derived mesenchymal stem cells (BM-MSCs) have shown promising results [6, 7], some reports have suggested that aging and disease reduce the quantity and quality of stem cells [8, 9]. In addition, the invasive process of bone marrow aspiration, the risk of viral contamination, and the limited availability of bone marrow donors may also limit the application of BM-MSCs transplantation [10]. At present, umbilical cord-derived mesenchymal stromal cells (UC-MSCs) draw much attention because they can avoid these disadvantages of BM-MSCs. Until now, many clinical studies used UC-MSCs to treat graft-versus-host disease [11], cirrhosis [12], or diabetes mellitus [13]. A recent clinical study used UC-MSCs to treat acute myocardial infarction (AMI) and demonstrated that intracoronary delivery of UC-MSCs was safe and could significantly improve myocardial viability and heart function [14]. However, there are as yet no data about the effect of UC-MSCs on chronic myocardial ischemia.
In this study, we investigated the possible therapeutic effect of UC-MSCs originated from Wharton’s jelly in the treatment of chronic myocardial ischemia in a porcine model. To facilitate the therapeutic effect, we used a combined cell delivery method by intracoronary administration combined with two intravenous infusions.
Materials and Methods
This study was conducted according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and all experiments were performed in accordance with the Helsinki Declaration. The animals were obtained from the Institute of Zoology, Chinese Academy of Sciences and were housed at the Bei Jing Tong He Sheng Tai Institute of Comparative Medicine. The experimental protocol was approved by the Animal Care and Use Committee of the Bei Jing Tong He Sheng Tai Institute of Comparative Medicine.
Study Design
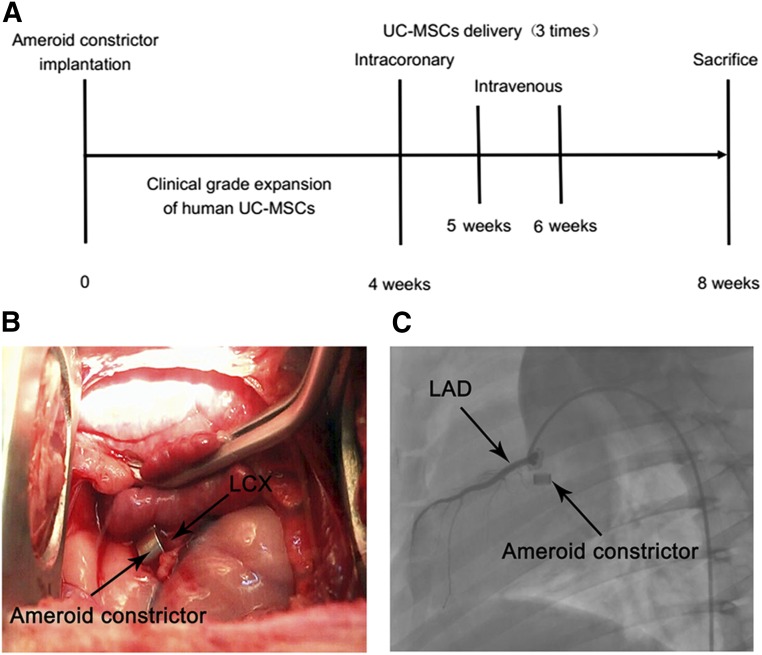
The summary of the study design is shown in Figure 1A. A total of 15 female pigs, weighing between 30 and 35 kg, underwent open-chest surgery, followed by placement of an ameroid constrictor to induce ischemia (day 0, Fig. 1A). Four weeks after surgery, pigs were randomly assigned into two groups: a control group and a cell transplantation group. All pigs underwent functional measurements including electrocardiogram, echocardiography, coronary angiography, and single photon emission computed tomography (SPECT). UC-MSCs (cell transplantation group, 30 × 106 in 15 ml of sterile saline) or saline (control group, 15 ml of sterile saline) were infused into the left main coronary artery by coronary angiography. Later, at the fifth and sixth week after surgery, UC-MSCs (cell transplantation group, 30 × 106 in 30 ml of sterile saline) or saline (control group, 30 ml of sterile saline) were infused intravenously twice through an ear vein catheter. Eight weeks after surgery, all pigs underwent functional measurements again before being humanely killed for histopathologic analyses of the hearts.
Figure 1.
Study design and porcine model preparation of chronic myocardial ischemia. (A): Study design. (B): An ameroid constrictor was placed around the left circumflex artery (LCX) to create a porcine model of chronic myocardial ischemia. (C): Four weeks after the implantation of the ameroid constrictor, left coronary angiography images showed complete occlusion of the LCX. Abbreviations: LAD, left anterior descending artery; UC-MSCs, umbilical cord-derived mesenchymal stromal cells.
Chronic Ischemia Model
Pigs were preanesthetized by an intramuscular injection of ketamine (25 mg/kg) and diazepam (1 mg/kg) [15]. After endotracheal intubation, inhalation anesthesia was maintained with isoflurane (0%–5%) and oxygen. The endotracheal tube was connected to a volume-controlled mechanical ventilator. The pig underwent a left thoracotomy, and the pericardium was dissected to expose the left circumflex coronary artery (LCX). An ameroid constrictor (2.5 mm; Research Instruments NW, Lebanon, OR, http://researchinstrumentsnw.com) was placed around the proximal portion of the artery (Fig. 1B), the pericardium was closed, and the thoracotomy was closed after the air had been evacuated from the thoracic cavity. After surgery, the animals were treated with 3.2 × 106 U benzylpenicillin sodium by an intramuscular injection for 3 consecutive days.
Cell Preparation
Human umbilical cord was aseptically collected from cesarean section following approval of the informed consent guidelines by the Ethical Review Board of the Affiliated Hospital of Academy of Military Medical Sciences. Written informed consent was obtained from the puerpera. UC-MSCs were prepared and expanded as previously described [16]. The umbilical cord Wharton’s jelly tissue was digested by hyaluronidase (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and Amnion/UC Collagenase (Sigma-Aldrich). Then the digestion was centrifuged and washed with phosphate-buffered saline (PBS). Finally, the cells were suspended in FasGrow medium (Bai Le Tong, Beijing, People’s Republic of China) and were incubated at 37°C in humidified 95% air and 5% CO2. Cells were subcultured once they attained 80% confluence. UC-MSCs in passage 5 were used for experiments.
To characterize UC-MSCs, the cultured cells were differentiated along adipogenic, osteogenic, and chondrogenic lineages as previously described [17] and analyzed by flow cytometry for MSC markers [18]. For flow-cytometric analysis, the cells were stained with antibodies against CD29, CD34, CD44, CD45, CD73, CD90, CD105, HLA-ABC, and HLA-DR, which were conjugated with fluorescein isothiocyanate, phycoerythrin, and peridinin-chlorophyll-protein Complex (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com).
Before implantation into animal models, the UC-MSCs were labeled with the cross-linkable membrane dye CM-Dil (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) according to the manufacturer’s protocol and finally filtered with a 70-µm cell strainer.
Measurement of Cardiac Function by Echocardiography
To assess myocardial function, an echocardiogram was performed the day before UC-MSC transplantation and 4 weeks after transplantation (immediately before sacrifice) by using an M7 ultrasound system (Mindray Medical Systems, Shenzhen, People’s Republic of China, http://www.mindray.com) equipped with 2.0–3.6 MHz phased array transducers [19]. Left ventricular (LV) end systolic volume (ESV) and LV end diastolic volume (EDV) were obtained by using the Simpson double-plane method, and ejection fraction (EF) was calculated as follows: EF (%) = (EDV − ESV)/EDV × 100. B-mode (two-dimensional) images were acquired in the LV long-axis view at the middle level of the papillary muscle. LV end-diastolic diameter, LV end-systolic diameter, and infarct wall thickness were measured from B-mode images. Electrocardiography was performed at the same time. R-R was measured from the electrocardiogram. Heart rate (HR) was calculated as follows: HR (bpm) = 60/(R-R interval).
Perfusion Assessment With 99mTc-Methoxyisobutylisonitrile
Myocardial perfusion was evaluated by SPECT imaging immediately before UC-MSC transplantation and 4 weeks after transplantation. Rest-SPECT was performed 60 minutes after an intravenous injection of all of the 555 MBq 99mTc-Methoxyisobutylisonitrile (China Institute of Atomic Energy, Beijing, People’s Republic of China, http://www.ciae.ac.cn). Gated SPECT was performed with a double-head g-camera (Symbia T2; Siemens Medical Solutions, Malvern, PA, http://usa.healthcare.siemens.com). Semiquantitative visual interpretation was performed by using a 20-segment model. Each segment was scored using a 5-point scale as follows: 0 = normal, 1 = equivocal, 2 = moderate, 3 = severe reduction of radioisotope uptake, and 4 = apparent absence of detectable tracer uptake in a segment [20]. Thus, total scores of MIBI uptake were automatically calculated on the rest images. A summed rest score (SRS) was obtained by adding the scores of 20 segments of images. To assess the change in myocardial perfusion, a summed difference score (SDS) was calculated by subtracting post-treatment SRS from pretreatment SRS based on Quantitative Perfusion SPECT (QPS) software results. The extent of myocardial perfusion abnormalities (percent) was expressed relative to the LV based on polar maps.
Collateral Vessel Assessment With Coronary Angiography
Selective coronary angiography was performed after administration of a 3,000-IU intravenous bolus of heparin. Coronary artery stenosis was estimated visually by two independent observers who were blinded to pig identity and clinical information. Collateral vessels were graded according to the Rentrop classification: 0 = no filling of any collateral vessels, 1 = filling of side branches of the artery to be perfused by collateral vessels without visualization of the epicardial segment, 2 = partial filling of the epicardial artery by collateral vessels, and 3 = complete filling of the epicardial artery by collateral vessels [21].
Cell Implantation
Intracoronary Delivery
Four weeks after implanting the ameroid constrictor, pigs were anesthetized, and the right femoral artery was cannulated under fluoroscopic guidance. A 6F guiding catheter (Medtronic, Minneapolis, MN, http://www.medtronic.com) was used to engage the ostium of the left coronary artery. Either 30 × 106 human UC-MSCs in 15 ml of sterile saline or control solution without cells was injected slowly into the coronary artery within 5 min.
Intravenous Infusion
At the fifth and sixth weeks, two additional intravenous administrations of UC-MSCs were performed. The UC-MSCs (30 × 106) in 30 ml of normal saline were infused into an ear vein catheter at a rate of 2–3 ml/minute. Pigs in the control group were injected with 30 ml of saline at the same time point.
Myocardial Histopathological and Quantitative Polymerase Chain Reaction Analysis
After excision of the heart, the LV was isolated and cut into eight slices from the apex to the base [18]. The slices were embedded in paraffin or optimal cutting temperature compound (OCT) for further histopathological analysis, including hematoxylin and eosin, Masson’s Trichrome, von Willebrand factor (vWF) immunostaining, and terminal deoxynucleotide transferase-mediated (dUTP) nick end-labeling (TUNEL) assay.
Angiogenesis Evaluation
Angiogenesis was evaluated in paraffin-embedded sections immunostained for vWF by using 3,3,N-diaminobenzidine tetrahydrochloride as a chromogen. Briefly, paraffin sections were cut into 5-µm-thick sections for immunohistochemical staining. Microvessels were identified by using a rabbit anti-vWF (Dako, Carpinteria, CA, http://www.dako.com) antibody. Microvessels positively stained by vWF were calculated from randomly selected microscopic fields by using computer-assisted morphometry. Capillary density was the average number of microvessels in 16 fields under ×400 magnification [2].
Apoptosis Assay
Apoptosis was evaluated by using the TUNEL assay kit (Roche, Indianapolis, IN, http://www.roche.com). The TUNEL-positive cells were counted in 16 different microscopic fields (under ×400 magnification) of at least four different sections for each animal. The percentage of apoptotic cells in the total number of cells examined was termed the apoptotic index [15].
Collagen Determination
To determine collagen volume fraction (CVF), four Masson’s Trichrome staining sections were used for every animal, and three low power fields were randomly selected in each section. The collagen volume was analyzed by using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, http://www.mediacy.com) by an independent investigator, who classified them according to the previously reported criteria. CVF was calculated as the area occupied by collagens divided by the total area [2].
Cell Implantation and Differentiation
Myocardial samples were snap-frozen in liquid nitrogen with Tissue-Tek OCT compound (Sakura, Tokyo, Japan, http://www.sakura-finetek.com) and were cut into 5-µm-thick slices. Before staining, frozen sections were fixed for 10 min in cold acetone. The sections were then incubated with antibodies at 4°C overnight against cardiac troponin I (cTn-I; 1:100; Abcam, Cambridge, MA, http://www.abcam.com), connexin-43 (Cx-43; l:100; Abcam), or vWF (l:100; Dako). After washing with PBS, Alexa 488-labeled IgG (green color; Thermo Fisher Scientific Life Sciences; 1:200 dilution) was added and incubated for 1 h at room temperature. Cell nucleus was counterstained with Hoechst 33258.
Quantitative mRNA Expression Analysis for Cytokines
The polymerase chain reaction (PCR) primers were as follows: vascular endothelial growth factor (VEGF), forward 5′-GAGTACCCCGATGAGATCGAGT-3′ and reverse 5′-GCTCATCTCTCCTATGTGCTGG-3′; angiopoietin (Ang), forward 5′-CGCGCCGCTCGACTAT-3′ and reverse 5′-CATGATATTCTCCAGCACTTGCA-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-CTGGCAAAGTGGACATTGTCGCCATC-3′ and reverse 5′-TTGCCGTGGGTGGAATCATACTGGAAC-3′. PCR amplification was performed on an Applied Biosystems StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) as follows: 10 minutes of initial denaturation at 95°C followed by 40 cycles of 15 seconds of denaturation at 95°C, and 30 seconds of annealing/extension at 60°C. Each sample was analyzed in duplicate. Analysis of relative quantification was performed as previously described [22].
Statistical Analysis
The data were expressed as the mean ± SEM. Comparisons of serial measurements in two groups were performed with two-way repeated measures analysis of variance (time and group). Post-treatment versus pretreatment and UC-MSC versus control comparisons were performed by using paired and unpaired Student’s t tests. A value of p < .05 was considered statistically significant. All analyses were performed by using SPSS software version 19.0 (IBM, Armonk, NY, http://www.ibm.com).
Results
Four weeks after ameroid constrictor placement, 12 pigs survived (80%) and had total LCX occlusion (Fig. 1C). During the period of the following study, no severe events, including malignant arrhythmias, bleeding, embolization, or hemodynamic deterioration, were observed, and the animals were in good condition.
Characterization of UC-MSCs
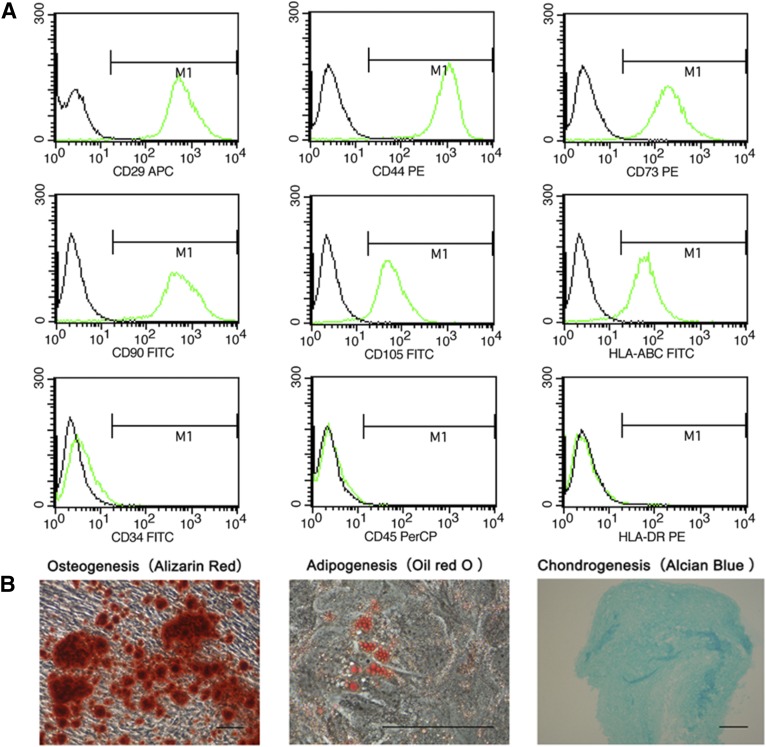
As shown in Figure 2A, UC-MSCs revealed the uniform expression of CD29, CD44, CD73, CD90, CD105, and HLA-ABC, but not of CD34, CD45, and HLA-DR. After multiple passages, the pluripotency of UC-MSCs was confirmed by the capacity to differentiate into adipogenic, osteogenic, and chondrogenic lineages in vitro (Fig. 2B).
Figure 2.
Characterization of human umbilical cord-derived mesenchymal stromal cells (UC-MSCs). (A): Representative flow cytometry histograms of human UC-MSCs. (B): Human UC-MSCs are positive for CD29, CD44, CD73, CD90, CD105, and HLA-ABC, and negative for CD34, CD45, and HLA-DR. Scale bars = 200 µm. Abbreviations: APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin-chlorophyll-protein complex.
UC-MSC Transplantation Improved LV Remodeling and Function
The HR and the echocardiographic parameters were similar in the control and UC-MSC group at pretreatment. At the 28-day follow-up, the HR increased significantly in the control group (p < .01), whereas in the UC-MSC group, there were no significant changes (Table 1). LV end diastolic volume (p < .01) and LV end systolic volume (p < .05) increased significantly in the control group when compared with pretreatment, whereas EDV and ESV in the UC-MSC group was almost unchanged (Table 1); this indicates that after UC-MSC transplantation, LV remodeling was alleviated.
Table 1.
Heart rate and echocardiography data before and after treatment
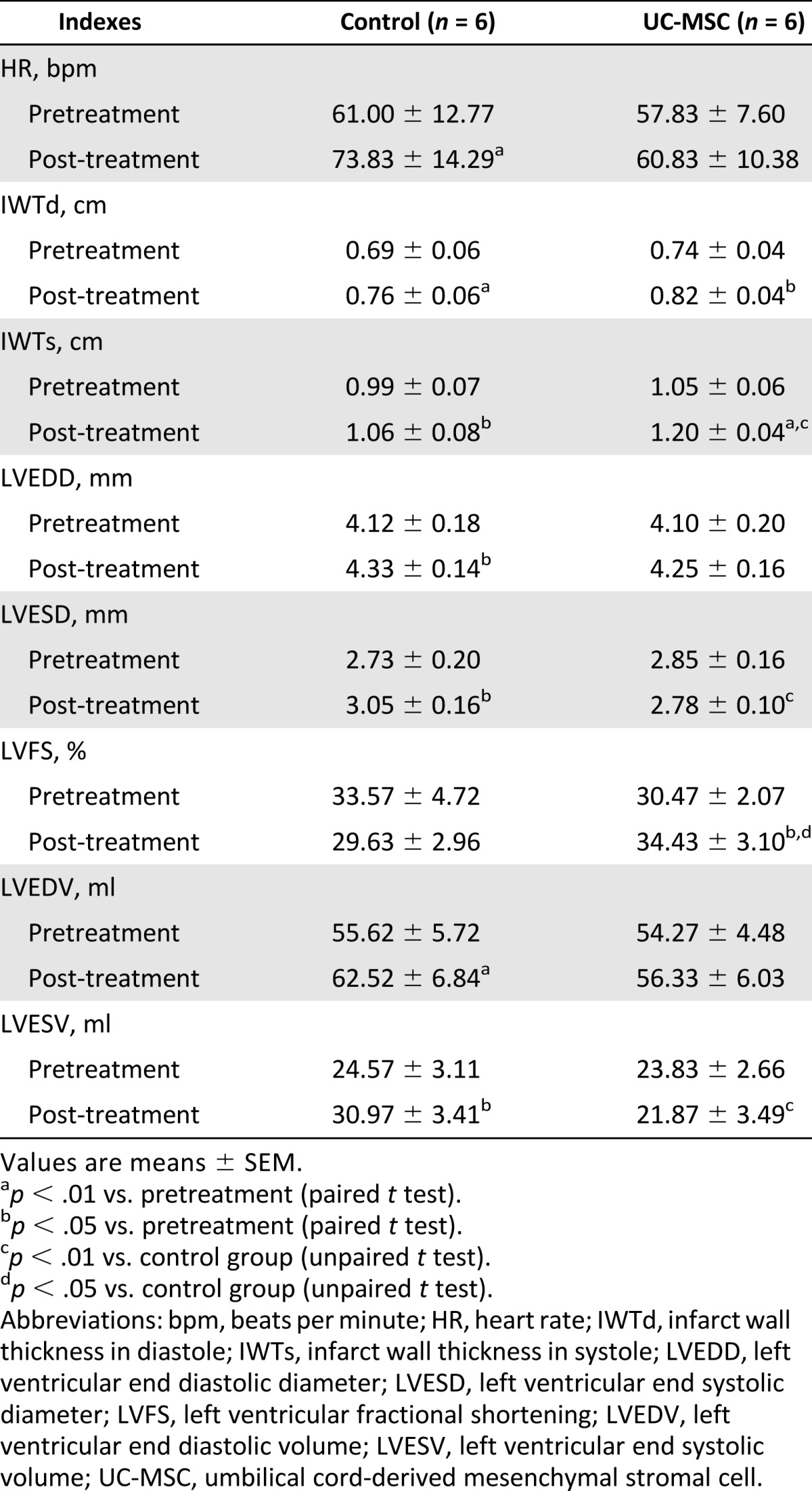
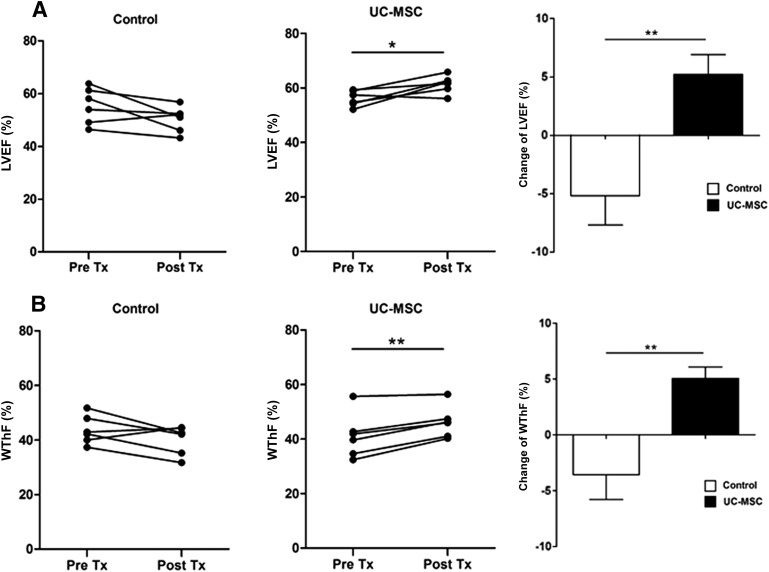
The EF assessed by echocardiography did not significantly differ between the two groups at pretreatment. However, after 4 weeks, it decreased in the control group by 5.2% ± 2.5% but improved by 5.2% ± 1.7% in the UC-MSC group (p < .01) (Fig. 3A). The change of the EF between the pretreatment and post treatment was statistically significantly different between the two groups (p < .01). Similarly, the LV fractional shortening also increased significantly in the UC-MSC group (Table 1). These findings indicate that UC-MSC transplantation improved global LV systolic function.
Figure 3.
Echocardiography assessment of global and regional LV function before and after UC-MSC therapy. (A): Time course changes in LVEF immediately before and 4 weeks after treatment. (B): Time course changes in the infarct wall thickening fraction. Left: Control group (n = 6). Middle: UC-MSC infusion group (n = 6). Right: differences between pretreatment and after 4 weeks. Data are means ± SEM. ∗, p < .05; ∗∗, p < .01. Abbreviations: LVEF, left ventricular ejection fraction; Post Tx, post treatment; Pre Tx, pretreatment; UC-MSC, umbilical cord-derived mesenchymal stromal cell; WThF, infarct wall thickening fraction.
The systolic thickening fraction in the infarcted wall (WThF) was similar in the control and UC-MSC groups before treatment. After treatment, however, it improved significantly in the UC-MSC group (p < .01) (Fig. 3B). WThF changes between the pretreatment and post-treatment was significantly different between the two groups (p < .01), indicating improved regional systolic function.
UC-MSCs Transplantation Increased Myocardial Perfusion and Collateral Vessels
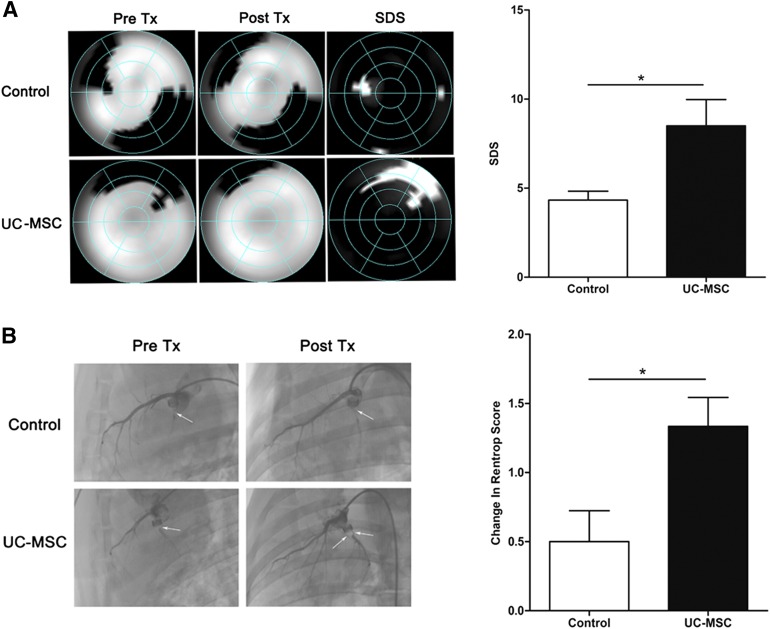
The alteration of myocardial perfusion was reflected by SDS, which was calculated by subtracting post-treatment SRS from pretreatment SRS based on QPS software results. Four weeks after treatment, SDS showed a significant improvement in the transplantation group compared with the control group (p < .05) (Fig. 4A), indicating that treatment with UC-MSC therapy significantly increased myocardial blood flow.
Figure 4.
Myocardial perfusion and collateral development assessed by coronary angiography and 99mTc-Methoxyisobutylisonitrile single photon emission computed tomography. (A): Representative images of myocardial perfusion before and after treatment and the improvement of myocardial perfusion after transplantation of UC-MSCs or normal saline (left). Right: The difference between post-treatment and pretreatment was compared by summed rest perfusion score. (B): Representative left coronary angiographic images before and after treatment (arrows show collaterals). Well-developed collaterals to the left circumflex artery were observed after the UC-MSC treatment (left). Right: Change in Rentrop score of collateral development in the control and UC-MSC-infusion groups. Data are means ± SEM. ∗, p < .05 versus control group (unpaired t test). Abbreviations: Post Tx, post treatment; Pre Tx, pretreatment; SDS, summed difference score; UC-MSC, umbilical cord-derived mesenchymal stromal cell.
Selective left coronary angiography was performed to evaluate collateral development before and after transplantation. The mean value of the Rentrop score of collateral development to the LCX territory at pretreatment was similar in the control and UC-MSC groups. After UC-MSC transplantation, Rentrop scoring was significantly improved (1.3 ± 0.2 vs. 2.6 ± 0.2, p < .01) but not in the control group (1.3 ± 0.2 vs. 1.8 ± 0.2, p > .05). The change in the Rentrop score was significantly greater in the UC-MSC group than in the control group (p < .05) (Fig. 4B). The results indicated that UC-MSCs promoted the collateral development.
UC-MSC Transplantation Promoted Angiogenesis, Reduced Apoptosis, and Fibrosis
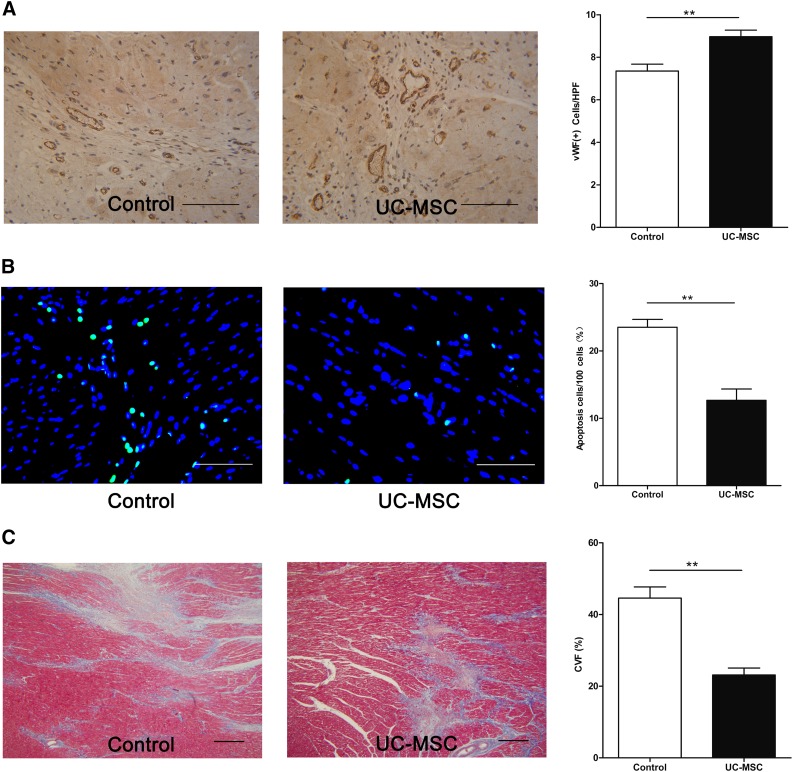
Immunohistochemical staining of vWF was performed to identify capillaries in the border areas 4 weeks after cell transplantation. Capillary density was significantly greater in the UC-MSC group than in the control group (p < .01) (Fig. 5A), indicating promoted angiogenesis.
Figure 5.
Assessment of angiogenesis, apoptosis, and fibrosis. (A): Representative images of the vWF-stained sections of the control and UC-MSC transplantation groups (left). Right: Quantification of vWF-positive capillaries in the border areas. Scale bars = 100 µm. (B): Representative images showed decreased apoptosis in the UC-MSC group (left). Right: The percentage of apoptotic cells in the total number of cells examined was termed the apoptotic index. Scale bars = 100 µm. (C): Representative Masson’s Trichrome-stained myocardial sections (left). Right: The morphometric analysis shows a marked reduction in fibrosis, a lower CVF in the UC-MSC group. Scale bars = 500 µm. ∗∗, p < .01 versus control group (unpaired t test). Abbreviations: CVF, collagen volume fraction; HPF, high power field; UC-MSC, umbilical cord-derived mesenchymal stromal cell; vWF, von Willebrand factor.
TUNEL staining indicated that the number of apoptotic cells in the border area was significantly reduced in the UC-MSC group (13 ± 4 cells per 100 cells) compared with the control group (24 ± 3 cells per 100 cells) (p < .001; Fig. 5B). This indicates that UC-MSC transplantation inhibited apoptosis of myocardial cells.
CVF evaluated by using the Masson's Trichrome-stained sections showed significantly less fibrosis in the hearts from the MSC-treated pigs than those from the control (44.6% ± 3.1% vs. 23.1% ± 2.0%, p < .01, Fig. 5C). These findings indicate that UC-MSC transplantation reduced fibrosis.
Engraftment and Differentiation of UC-MSCs
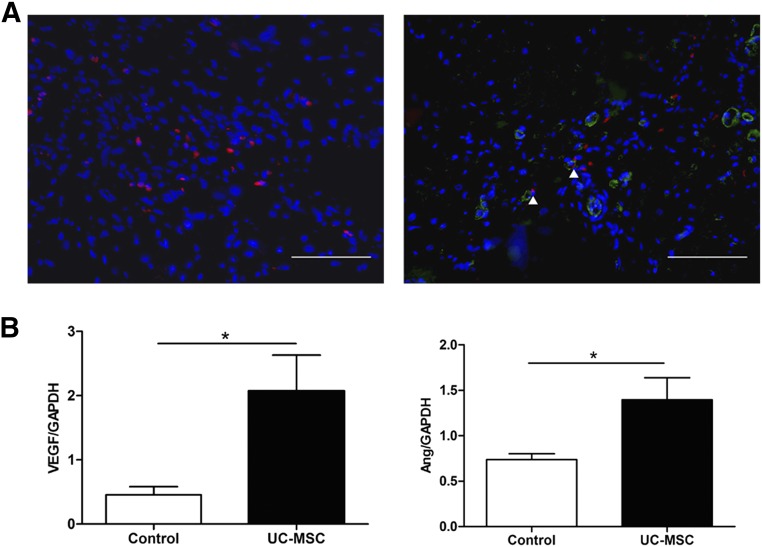
Before implantation into animal models, UC-MSCs were labeled with CM-Dil. At the end of the experiment, the engraftment and differentiation of the transplanted UC-MSCs were assessed under a fluorescent microscope. CM-Dil-positive cells were found in border areas, and a small number of them expressed endothelial marker vWF (Fig. 6A). However, the expression of the cardiomyocyte-specific marker cTnI or Cx43 was not observed (data not shown).
Figure 6.
MSC differentiation and paracrine effect. (A): Engraftment of UC-MSCs (left). Right: Endothelial cells differentiation of the engrafted UC-MSCs. Red fluorescence is CM-DiI labeling of UC-MSCs. Green fluorescence indicates vWF positive. Blue fluorescence indicates Hoechst 33258 nuclear staining. Scale bars = 100 µm. (B): Quantitative analysis of VEGF mRNA expression relative to GAPDH mRNA in the border area of the heart (left). Right: Quantitative analysis of Ang mRNA expression relative to GAPDH mRNA in the border area of the heart. ∗, p < .05 versus control group (unpaired t test). Abbreviations: Ang, angiopoietin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; UC-MSC, umbilical cord-derived mesenchymal stromal cell; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor.
UC-MSC Transplantation Enhanced Expression of Proangiogenic Cytokines
To examine whether the factors related to angiogenesis contributed to the neovascularization in the treated myocardium, we determined the gene expression levels of VEGF and Ang relative to GAPDH by quantitative reverse-transcription polymerase chain reaction analysis. The VEGF and Ang levels were significantly higher in the UC-MSC-treated hearts (p < .05; Fig. 6B). These findings indicated that transplanted human UC-MSCs may enhance neovascularization partly through paracrine effect.
Discussion
This is the first study to evaluate the feasibility and effectiveness of UC-MSCs for cellular cardiomyoplasty in a large animal model of chronic cardiac ischemia that closely reproduces the current clinical procedural management of myocardial infarction, including instrumentation used in humans. Four weeks after intracoronary administration combined with two subsequent intravenous injections of UC-MSCs, LV perfusion, function, and remodeling were substantially improved. Histologically, UC-MSC transplantation significantly decreased fibrosis and apoptosis and enhanced neovascularization. None of the animals in our cohort developed fatal arrhythmias or suffered from sudden death. This study demonstrated that UC-MSCs were feasible and effective for the treatment of CHD.
Among the numerous stem, progenitor, and mature cells that work beneficially in cardiac repair, MSCs could be the optimal cell type to be used clinically because of their safety and availability. Although most studies applied BM-MSCs, UC-MSCs were considered to be an ideal alternative to BM-MSCs because of its unique characters such as: (a) the extraction process (i.e., absence of invasiveness and high efficiency), (b) high cell yield, and (c) the short doubling time, enabling quick attainment of the abundant cell numbers required for clinical use while maintaining a safe profile. At present, there are a lot of experimental and clinical studies in using UC-MSCs in many kinds of diseases [11–13]. However, in heart disease, the studies are limited to AMI. Latifpour et al. subepicardially transplanted human umbilical cord mesenchymal cells (hUCMs) and 5-azacytidine-treated hUCMs to a rabbit model of AMI. After 30 days, they found that the EF and percentage of fractional shortening improved significantly in cell-receiving animals, and the amount of scar tissue was significantly reduced [23]. Zhang et al. reported that UC-MSCs can improve ventricular remodeling and function in a porcine model of AMI [24]. Recently, a clinical trial evaluated the effect of UC-MSCs in AMI and found that LVEF increased and LVESV and LVEDV decreased significantly [14]. However, until now, there has been no report about the use of UC-MSCs in the treatment of chronic heart ischemia. In this study, we delivered UC-MSCs by intracoronary administration combined with two subsequent intravenous administrations and found that LV perfusion, function and remodeling were substantially improved. Because UC-MSCs are isolated from medical waste without ethical controversy, are easily expanded to the large numbers of cells required for transplantation within a short period of time, and can be administered to patients at any time without immunological rejection, they have the potential to be a convenient and preferable stem cell source for cardiac therapy and may be used as an “off-the-shelf” strategy for allogeneic transfer.
To date, three main routes of cell delivery are used [25], including (a) intramyocardial, (b) intracoronary, and (c) intravenous. Each delivery technique has its own risks and benefits. Direct intramyocardial injection directly targets the injured myocardium but requires complicated devices and operating skills. Percutaneous coronary cell delivery is less invasive and demonstrates the most frequent application route in the clinical setting. Until now, controversy existed about whether intramyocardial or intracoronary administration was superior [26–28]. Some studies have shown that systemic intravenous administration of cells could hardly home to the heart because of the pulmonary first-pass effect. However, other studies declared that intravenous delivery of MSCs could limit infarct size and improve LV function, which could be a result of the trapped cells in the lung secreting TSG6, which is beneficial for revascularization of a damaged heart [29]. Until now, the overwhelming majority of stem cell treatment studies in heart disease used a single delivery method. Sürder et al. hypothesized that a combined cell-delivery approach (intramyocardial and intracoronary) is safe and effective [30]. In this study, we combined intracoronary delivery with multiple intravenous infusions. The results showed that the cardiac function improved, the perfusion increased, and ventricular remodeling was alleviated, thus demonstrating the efficiency of the combined delivery methods. Both intracoronary delivery and intravenous infusion are convenient in clinical practice, but whether the combination of both is more superior than one alone still needs further examination.
It has been reported that the mechanisms of MSC action on myocardial regeneration may arise from their direct transdifferentiation into cardiac cell lineages, paracrine actions via cytokine secretion, niche optimization for residential cardiac stem cells, or inflammatory control [31]. In this work, we observed transplanted cells in the ischemic heart; however, immunohistochemical analysis revealed that only a small number of the implanted cells differentiated into endothelial cells. Although transdifferentiation into cardiomyocytes has been reported previously with BM-MSCs [32], no cardiomyocyte differentiation of the transplanted cells was observed in this study. Therefore, we speculate that the therapeutic effect of UC-MSCs may be essentially exerted through a paracrine mechanism. We detected the increase of VEGF and Ang mRNA in the border zone of the ischemic heart, which indicated that the angiogenesis may contribute to the therapeutic effect of UC-MSC transplantation.
Conclusion
This is the first study to provide evidence that intracoronary delivery combined with multiple intravenous infusions of UC-MSCs improves LV function, perfusion, and remodeling in a large animal model of chronic myocardial ischemia. In the present study, we observed neither tumor nor teratoma formation in human UC-MSC-transplanted animals, and no sustained ventricular arrhythmia or anaphylaxis was observed. Because these cells can be isolated from medical waste, expanded, banked, and administered to patients at any time without immunological rejection, human UC-MSCs might be an ideal cell source for cardiac cell therapy and hold promise as an off-the-shelf product.
Acknowledgments
This study was supported in part by National Natural Science Foundation of China Grants 81370210 and 31470940. We thank Wei-Wei Liu for statistical contributions; Jun Li, Can Jin, Shi-Feng Ning, Cui-Hong Zhao, Xiu-Li Zhang, Wei-Bin Cheng, Mei-Chao Guan, Xiang-Yun Rong, Qiong Hu, Yue Li for their technical contributions; and Bei Jing Tong He Sheng Tai Institute of Comparative Medicine for technical assistance in animal surgery.
Author Contributions
C.-B.L.: conception and design, organization of the whole experiment, data analysis and interpretation, manuscript writing, final approval of manuscript; H.H.: data collection and interpretation, financial support, manuscript writing, final approval of manuscript; P.S. and S.-Z.M.: collection and/or assembly of data, final approval of manuscript; A.-H.L., J.X., and J.-H.F.: participated in surgery, coronary angiography, echocardiography, single photon emission computed tomography, final approval of manuscript; Y.-Q.L.: cell culture, collection and/or assembly of data, final approval of manuscript; B.L.: instruction of laboratory examination, administrative support, final approval of manuscript; D.-Y.W.: instruction of UC-MSC preparation and examination, histopathological examination, final approval of manuscript; S.-H.L.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript; X.-Z.Z.: conception and design, surgery of model preparation and UC-MSC transplantation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Giusti II, Rodrigues CG, Salles FB, et al. High doses of vascular endothelial growth factor 165 safely, but transiently, improve myocardial perfusion in no-option ischemic disease. Hum Gene Ther Methods. 2013;24:298–306. doi: 10.1089/hgtb.2012.221. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, Iso Y, Uyama T, et al. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab Invest. 2011;91:553–564. doi: 10.1038/labinvest.2010.202. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Tang XL, Sanganalmath SK, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assmus B, Leistner DM, Schächinger V, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: Migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35:1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 5.van Ramshorst J, Rodrigo SF, Schalij MJ, et al. Bone marrow cell injection for chronic myocardial ischemia: The past and the future. J Cardiovasc Transl Res. 2011;4:182–191. doi: 10.1007/s12265-010-9249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams AR, Suncion VY, McCall F, et al. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J Am Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 9.Heiss C, Keymel S, Niesler U, et al. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 10.Vassalli G, Moccetti T. Cardiac repair with allogeneic mesenchymal stem cells after myocardial infarction. Swiss Med Wkly. 2011;141:w13209. doi: 10.4414/smw.2011.13209. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 13.Chao KC, Chao KF, Fu YS, et al. Islet-like clusters derived from mesenchymal stem cells in Wharton’s jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao LR, Chen Y, Zhang NK, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015;13:162. doi: 10.1186/s12916-015-0399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L, Yang YJ, Dong QT, et al. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS One. 2013;8:e65702. doi: 10.1371/journal.pone.0065702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montanucci P, Basta G, Pescara T, et al. New simple and rapid method for purification of mesenchymal stem cells from the human umbilical cord Wharton jelly. Tissue Eng Part A. 2011;17:2651–2661. doi: 10.1089/ten.TEA.2010.0587. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M, Toyoda M, Gojo S, et al. Allogeneic amniotic membrane-derived mesenchymal stromal cell transplantation in a porcine model of chronic myocardial ischemia. J Stem Cells Regen Med. 2012;8:171–180. doi: 10.46582/jsrm.0803010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muzzarelli S, Pfisterer ME, Müller-Brand J, et al. Interrelation of ST-segment depression during bicycle ergometry and extent of myocardial ischaemia by myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2009;36:1842–1850. doi: 10.1007/s00259-009-1167-0. [DOI] [PubMed] [Google Scholar]
- 21.Abaci A, Oğuzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Latifpour M, Nematollahi-Mahani SN, Deilamy M, et al. Improvement in cardiac function following transplantation of human umbilical cord matrix-derived mesenchymal cells. Cardiology. 2011;120:9–18. doi: 10.1159/000332581. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Liu XC, Yang L, et al. Wharton’s jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coron Artery Dis. 2013;24:549–558. doi: 10.1097/MCA.0b013e3283640f00. [DOI] [PubMed] [Google Scholar]
- 25.Templin C, Lüscher TF, Landmesser U. Cell-based cardiovascular repair and regeneration in acute myocardial infarction and chronic ischemic cardiomyopathy-current status and future developments. Int J Dev Biol. 2011;55:407–417. doi: 10.1387/ijdb.103219ct. [DOI] [PubMed] [Google Scholar]
- 26.Perin EC, Silva GV, Assad JA, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 28.van der Spoel TI, Vrijsen KR, Koudstaal S, et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: A study on delivery efficiency. J Cell Mol Med. 2012;16:2768–2776. doi: 10.1111/j.1582-4934.2012.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sürder D, Radrizzani M, Turchetto L, et al. Combined delivery of bone marrow-derived mononuclear cells in chronic ischemic heart disease: Rationale and study design. Clin Cardiol. 2013;36:435–441. doi: 10.1002/clc.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou SH, Lin SZ, Kuo WW, et al. Mesenchymal stem cell insights: Prospects in cardiovascular therapy. Cell Transplant. 2014;23:513–529. doi: 10.3727/096368914X678436. [DOI] [PubMed] [Google Scholar]
- 32.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]