Translation of a robust differentiation protocol capable of producing scalable quantities of red blood cells (RBCs) from human pluripotent stem cells to the clinic was compromised because the RBCs produced were not fully mature, expressed embryonic and fetal globins, and did not enucleate efficiently. Programming with HOXB4 increased hematopoietic progenitor and immature erythroid cell production but could not resolve the inherent challenges associated with mature adult-like enucleated RBC production.
Keywords: CD34+, Cell surface markers, Colony formation, Differentiation, Electroporation, Embryonic stem cells, Erythroid differentiation, Transcription factor
Abstract
We have developed a robust, Good Manufacturing Practice-compatible differentiation protocol capable of producing scalable quantities of red blood cells (RBCs) from human pluripotent stem cells (hPSCs). However, translation of this protocol to the clinic has been compromised because the RBCs produced are not fully mature; thus, they express embryonic and fetal, rather than adult globins, and they do not enucleate efficiently. Based on previous studies, we predicted that activation of exogenous HOXB4 would increase the production of hematopoietic progenitor cells (HPCs) from hPSCs and hypothesized that it might also promote the production of more mature, definitive RBCs. Using a tamoxifen-inducible HOXB4-ERT2 expression system, we first demonstrated that activation of HOXB4 does increase the production of HPCs from hPSCs as determined by colony-forming unit culture activity and the presence of CD43+CD34+ progenitors. Activation of HOXB4 caused a modest, but significant, increase in the proportion of immature CD235a+/CD71+ erythroid cells. However, this did not result in a significant increase in more mature CD235a+/CD71− cells. RBCs produced in the presence of enhanced HOXB4 activity expressed embryonic (ε) and fetal (γ) but not adult (β) globins, and the proportion of enucleated cells was comparable to that of the control cultures. We conclude that programming with the transcription factor HOXB4 increases the production of hematopoietic progenitors and immature erythroid cells but does not resolve the inherent challenges associated with the production of mature adult-like enucleated RBCs.
Significance
As worldwide blood donations decrease and transfusable transmitted infections increase, intense interest has ensued in deriving red blood cells (RBCs) in vitro from alternative sources such as pluripotent stem cells. A translatable protocol was developed to generate RBCs; however, these RBCs have an immature phenotype. It was hypothesized that the transcription factor HOXB4 could enhance their production and maturation. Although HOXB4 increased the production of erythroid progenitors, it did not promote their maturation. Despite the remaining challenges, a robust system has been established to test other candidates and add to the knowledge base in this field.
Introduction
Cell-based therapies such as bone marrow transplantation and blood transfusion are used to treat diseases of the hematopoietic system; however, these procedures are completely reliant on a limited supply of donor tissue. Thus, one goal has been to produce therapeutic hematopoietic cells from a bankable and limitless source of human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) [1–3]. Many studies have been reported on the production of hematopoietic progenitors cells (HPCs) and mature hematopoietic cells from ESCs and iPSCs; however, it has been widely acknowledged that significant challenges exist in this field. The differentiation protocols that have been used to date are relatively inefficient, and it has proved particularly difficult to produce the most potent hematopoietic stem cells (HSCs) capable of long-term reconstitution (LTR-HSCs) and fully mature red blood cells (RBCs) that express adult β-globin and enucleate [4–6]. Most of the strategies used to produce erythroid cells have required the use of stromal cell feeder layers and/or ill-defined serum, which would present significant safety issues in clinical translation [7–11]. Feeder-free hematopoietic differentiation strategies have been developed, but many of these required lengthy embryoid body stages that have proved difficult to control and replicate effectively [8, 12, 13]. The production of fully mature adult-like erythroid cells that undergo appropriate globin switching has been particularly challenging, with most studies detecting embryonic (ζ, ε) and fetal (γ) but not adult (β) globin [8, 11, 14]. Although some studies have reported the production of some enucleated cells, none of these have demonstrated a convincing and robust degree of enucleation in pluripotent stem cell-derived RBCs [9, 12].
The first wave of erythropoiesis in vivo occurs in the yolk sac and is responsible for the production of primitive nucleated erythrocytes. A second wave initially arises in the aorta-gonad-mesonephros region, where the first definitive HSCs arise that are capable of long-term multilineage reconstitution and production of adult nucleated erythrocytes [15]. We hypothesized that the limited production of nucleated erythrocytes that can be achieved from pluripotent stem cells in vitro might reflect the “primitive” state of the progenitors produced in this system. This is supported by the finding that the production of LTR-HSCs in vitro from pluripotent stem cells capable of multilineage long-term reconstitution has proved particularly difficult to achieve. We reasoned that HOXB4 might be a transcription factor that could program hPSC-derived hematopoietic cells to a more “definitive” phenotype, because it has been shown to confer long-term reconstitution ability on primitive yolk sac cells and mouse ESCs [16–18]. Furthermore, our studies of the murine ESC system have demonstrated that HOXB4 could promote a paracrine effect; thus, its actions could mimic the action of stromal cell coculture [19–21]. The effects of exogenous HOXB4 on human ESCs has been less clear, with contradictory outcomes likely related to the range of the differentiation protocols used and the different expression strategies used, leading to variations in the level and timing of expression [19]. In the present study, we used a controllable HOXB4 expression system in which we could activate HOXB4 at different time points in a well-defined differentiation protocol.
We have developed a stepwise, serum- and feeder-free clinical grade protocol for both the growth and the maintenance of hESCs and hiPSCs and their subsequent expansion and differentiation into erythroid cells [22]. We tracked the emergence of HPCs using colony-forming unit culture (CFU-C) and flow cytometry and then tested the effects of tamoxifen-inducible expression of HOXB4 in this defined and robust hematopoietic differentiation protocol. In keeping with the results from murine studies, we found that enforced expression of HOXB4 resulted in a specific increase in the production of multilineage HPCs. We also noted a modest increase in the production of immature erythroid cells, but, overall, no significant effect was found for their subsequent maturation. The erythroid cells that were produced in the presence of HOXB4 failed to undergo efficient enucleation and expressed embryonic (ε) and fetal (γ), but not adult (β), globin. Thus, enforced expression of HOXB4 did not overcome the major difficulty in the production of mature enucleated cells required for transfusion.
Materials and Methods
Maintenance and Differentiation of hESCs
Human pluripotent stem cell lines, including H1 [23] and Runx1C-GFP [24], were routinely maintained in StemPro hESC serum-free medium on CELLstart matrix (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) with the addition of basic fibroblast growth factor (bFGF) 20 ng/ml (PHG0021; Thermo Fisher Scientific Life Sciences), and passaged (1:4) when 70%–80% confluent, using StemPro EZPassage tools (Thermo Fisher Scientific Life Sciences). Differentiation was performed in a stepwise, serum- and feeder-free protocol, as described [22]. In brief, one confluent well of human ESCs was cut and transferred to two wells (six-well plates; cell repellant surface; catalog no. 657970; Greiner Bio-One, Kremsmünster, Austria, http://www.greinerbioone.com) and then cultured in 3 ml per well of Stemline II Hematopoietic Stem Cell Expansion Medium (catalog no. S0192; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) with the addition of bone morphogenetic protein 4 (BMP4) 10 ng/ml (catalog no. 314-BP-010; R&D Systems, Minneapolis, MN, http://wwwrndsystems.com), vascular endothelial growth factor (VEGF) 10 ng/ml (catalog no. 293-VE010; R&D Systems), Wnt3a 10 ng/ml (catalog no. 5036-WN010; R&D Systems), activin A 5 ng/ml (catalog no. 338-AC010; R&D Systems), and glycogen synthase kinase (GSK-3β) inhibitor VIII 2 μM (catalog no. A014418; Merck Millipore, Billerica, MA, http://www.merckmillipore.com). EBs formed spontaneously, and after 2 days, the following cytokines were added in 500 μl of Stemline II per well: BMP4 20 ng/ml, VEGF 30 ng/ml, Wnt3a 10 ng/ml, activin A 5 ng/ml, fibroblast growth factor-α (FGF-α) 10 ng/ml (catalog no. PHG0014; Thermo Fisher Scientific Life Sciences), stem cell factor (SCF) 20 ng/ml (catalog no. PHC 2111; Thermo Fisher Scientific Life Sciences), and GSK inhibitor VIII 2 μM. EBs were disaggregated on day 3 with 0.5 ml per well StemPro Accutase Cell Dissociation Reagent for 3 minutes at 37°C, before gently pipetting 10 times and adding prewarmed medium. After centrifugation, the cells were replated at 2 × 105 cells per well (six-well plates; Corning, Corning, NY, http://www.corning.com) in 3 ml of Stemline II, with the addition of BMP4 20 ng/ml, VEGF 30 ng/ml, FGF-α 10 ng/ml, SCF 30 ng/ml, insulin-like growth factor 2 (IGF2) 10 ng/ml (catalog no. 292-G2; R&D Systems), thrombopoietin (TPO) 10 ng/ml (catalog no. 288-TPN-25; R&D Systems), heparin 5 μg/ml (catalog no. H3149; Sigma-Aldrich), and isobutylmethylxanthine (IBMX) 100 μM (catalog no. I5879; Sigma-Aldrich). On day 7, the floating cells and medium were carefully removed and centrifuged before replating all the cells back into the same dishes (containing any adherent cells) in 3 ml of fresh Stemline II medium containing BMP4 20 ng/ml, VEGF 30 ng/ml, FGF-α 10 ng/ml, SCF 30 ng/ml, IGF2 10 ng/ml, TPO 10 ng/ml, heparin 2.5 μg/ml, and IBMX 100 μM. On day 9, the same day-7 cytokines were topped up in 500 μl of Stemline II per well. On day 10, all the cells were aspirated from the well, centrifuged, and placed in 500 μΜ Accutase solution 37°C for 3 minutes, and 3 × 105 cells were replated per well in IBIT medium (240 ml of Iscove Basal Medium; catalog no. FG-0465; Biochrome, Merck Millipore), 1% bovine serum albumin (BSA; catalog no. G10008-01; Thermo Fisher Scientific Life Sciences), 10 μg/ml insulin (catalog no. I9278; Sigma-Aldrich), 0.2 mg/ml transferrin (catalog no. T0665; Sigma-Aldrich), and 500 μl β-mercaptoethanol (catalog 31550-010; Thermo Fisher Scientific Life Sciences), with the addition of hydrocortisone 10 μM, SCF 50 ng/ml, FMS-like tyrosine kinase receptor 3 ligand (Flt3L) 16.7 ng/ml (catalog no. 300-19; PeproTech, Rocky Hills, NJ, http://www.peprotech.com), BMP4 6.7 ng/ml, interleukin (IL)-3 6.7 ng/ml (catalog 213-13; PeproTech), IL-11 6.7 ng/ml (catalog no. 200-11; PeproTech), erythropoietin (EPO) 3 U/ml (catalog no. 287-TC-500; R&D Systems), and IBMX 100 μM. Every 2 days, the cytokines were topped up in a volume of 500 µl per well. On day 17, the cells were centrifuged and counted, and 1 × 106 cells were plated per well in IBIT with the addition of hydrocortisone 10 μM, SCF 20 ng/ml, IGF1 20 ng/ml (catalog no. 100-11; PeproTech), IL-3 6.7 ng/ml, IL-11 6.7 ng/ml, and EPO 3 U/ml. The cytokines were replenished at the same concentrations every 2 days in a volume of 500 μl per well.
Flow Cytometry
The cells were harvested, and 2 × 105 cells were placed in aliquots in phosphate-buffered saline (PBS) containing 1% BSA (PBS/BSA) and centrifuged (200g) for 5 minutes. Antibodies were added directly to cell pellets that were then resuspended to a final volume of 100 μl, incubated for 30 minutes on ice, washed in 5 ml PBS/BSA, and resuspended in 300 μl. The samples were analyzed using an LSRFortessa (BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com) using FACSDiva acquisition (BD Biosciences) and FlowJo analysis software (FlowJo, Ashland, OR, http://www.flowjo.com), or sorted on a FACSARIA (BD Biosciences). Anti-human antibodies included CD71-FITC (catalog no. 11-0719; eBioscience, San Diego, CA, http://www.ebioscience.com), CD43-APC (catalog no. 17-0439-42; eBioscience), glycophorinA (M) (CD235a), efluor450 (catalog no. 48-9884; eBioscience), CD34-PE (catalog no. 12-0349; eBioscience), CD41a-FITC (BD Biosciences), CD45-V450 (BD Biosciences), and CD144-PE (Beckman Coulter, Brea, CA, http://www.beckmancoulter.com). Mouse IgG1 APC isotype control (catalog no. 17-4714-81; eBioscience) and mouse IgG1 PE isotype control (catalog no. 12-4714-81; eBioscience) were used (supplemental online Fig. 2).
CFU-C Assay
Single cells (5 × 103 or 104) were plated into 1.5 ml of Methocult medium containing SCF, granulocyte colony-stimulating factor (CSF), granulocyte macrophage CSF, IL-3, IL-6, and erythropoietin (StemCell Technologies, Inc., Vancouver, BC, Canada, http://www.stemcell.com) in 35-mm-dish low-attachment plates (Greiner Bio-One), incubated at 37°C in a humid chamber, and scored for hematopoietic colony formation after 12–15 days.
Magnetically Activated Cell Sorting
Magnetically activated cell sorting (MACS) was performed according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). In brief, the cells were resuspended in PBS containing 2 mM EDTA, incubated in FC blocking agent for 10 minutes at room temperature, combined with microbead-labeled antibody (Miltenyi Biotec; CD43:130-091-333 or CD34:130-046-702) for 20 minutes at 4°C, then washed and resuspended in PBS/EDTA. Labeled cells were then applied in 250-μl aliquots to the MACS column, left to stand in the magnet for 5 minutes, and then washed three times with 0.5 ml PBS/EDTA. The cells were released from the column according to the manufacturer’s instruction, and the purity of sorted cells was assessed by flow cytometry.
hESC Transfection
H1 hESCs were fed with fresh StemPro medium containing 20 ng/ml bFGF (R&D Systems) and 10 μM ROCK inhibitor (catalog no. Y-27632; Calbiochem, EMD Millipore, Billerica, MA, http://www.emdmillipore.com) at least 1 hour before electroporation, as described previously [25]. Single cell suspensions were generated using Accutase, then the cells were washed and resuspended in StemPro medium (107 cells per 0.5 ml) with 30 μg of linearized pCAG-HOXB4ERT2-IRES-PURO vector [20]. The cells were electroporated (Gene Pulser Xcell: 320 V, 250 μFd; Bio-Rad, Hercules, CA, http://www.bio-rad.com), plated on CELLstart matrix (Thermo Fisher Scientific Life Sciences) in StemPro medium containing 20 ng/ml bFGF (R&D Systems) and 10 μM ROCK inhibitor (catalog no. Y-27632; Calbiochem). Resistant colonies were selected in 0.6 μg/ml puromycin for 10 days, then single colonies were picked, expanded, and screened for expression of HOXB4 by Western blot (supplemental online Fig. 1).
High-Performance Liquid Chromatography
High-performance liquid chromatography (HPLC) globin chain separation was performed using a protocol modified from Lapillonne et al. [9]. Erythroid cells were washed three times in PBS, lysed in 50 μl of 0.1% trifluoroacetic acid (TFA) in water. The cells were then centrifuged at 13,000g at 4°C for 10 minutes, and the supernatant was collected for HPLC analysis. Globin chain separation was performed by injecting 10 μl of the supernatant onto a 1.0 × 250-mm C4 column (Phenomenex, Macclesfield, U.K., http://www.phenomenex.com) with a 42%–56% linear gradient between mixtures of 0.1% TFA in water and 0.1% TFA in acetonitrile at a flow rate of 0.05 ml per minute for 50 minutes on a HPLC Ultimate 3000 system (Dionex, Thermo Fisher Scientific Life Sciences). The column temperature was fixed at 50°C during analysis, and the UV detector was set at 220 nm. Lysates from adult peripheral blood and fetal liver were used as positive controls. The elution times of the peaks generated were compared with those of the control peaks for identification. The area under the curve was used to calculate the proportion of each globin peak from each sample.
Results
Monitoring Hematopoietic Progenitor Production
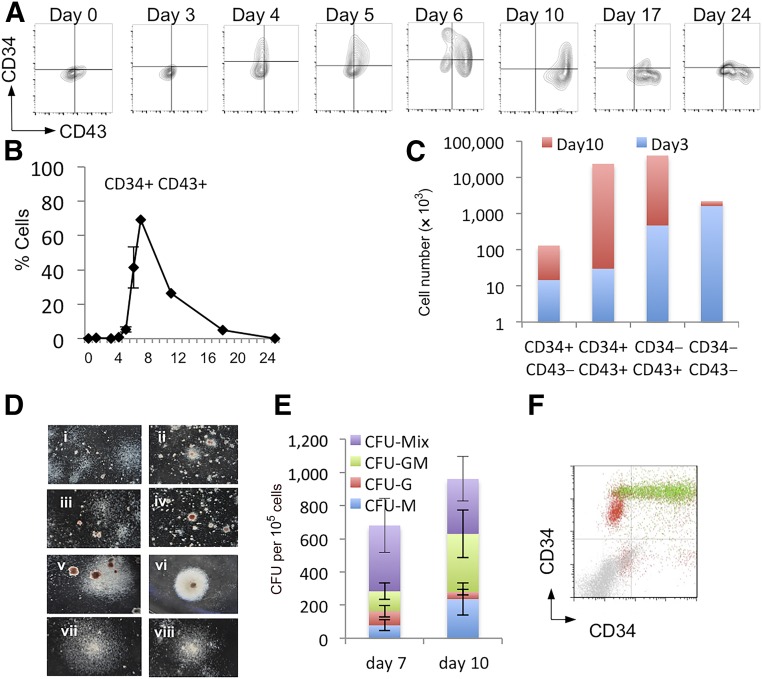
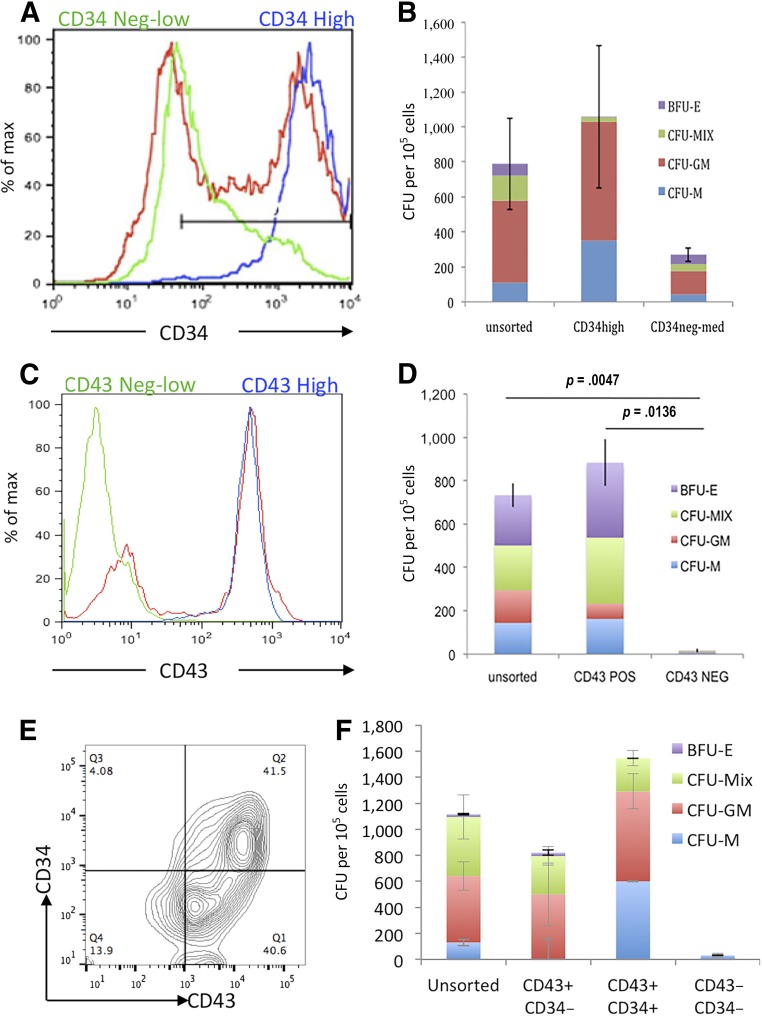
We monitored the production of HPCs throughout the differentiation protocol by flow cytometry and CFU-C assays (Fig. 1). The proportion of cells expressing CD34 and CD43 peaked between day 6 and 10 (Fig. 1A, B), and the appearance of cells carrying these HPC markers coincided with the presence of CFU-C colonies (Fig. 1E). Most CFU-C generated from the day 7 cells were small, primitive-like colonies (Fig. 1Di–3Div) with larger, more robust colonies produced by cells in the day 10 cultures (Fig. 1Dv–1Dviii). The absolute numbers of the CD34+ and CD43+ populations within the cultures at days 3 and 10 were calculated. The most abundant population at day 10 was the CD34+/CD43+ double-positive population (Fig. 1C). To further characterize the HPCs generated in our differentiation protocol, we used the RUNX1CGFP/w hESC line carrying a green fluorescent protein (GFP) reporter under the control of the endogenous Runx1C promoter [24], which is considered to be a marker of definitive hematopoietic stem cells [26]. Most of the double-positive, CD34+CD43+ cells at day 10 also expressed Runx1C-GFP, confirming their definitive progenitor-like phenotype (Fig. 1F). To determine which cell population contained the hematopoietic activity, we used MACS to differentiate ESCs at day 7 according to CD34 expression (Fig. 2A) and at day 10 according to CD43 expression (Fig. 2C) and then analyzed the enriched populations for hematopoietic colony activity (Fig. 2B, 2D). The maximum number of CFU-Cs was found within the CD34hi population at day 7 and CD43+ population at day 10. Further analyses of the fluorescence activated cell-sorted day 10 cells demonstrated that CFU-C activity was contained within the CD43+CD34+ and the CD43+CD34− (Fig. 2E, 2F).
Figure 1.
Monitoring hematopoietic progenitor production throughout the differentiation protocol. Representative flow cytometry analysis of cells from days 0 to 24 of the differentiation protocol using antibodies against CD34 and CD43 (A) and the quantification of the percentage positive (B) was performed from at least three independent experiments. Absolute cell numbers of the differentiation represented in (A) are shown for days 3 and 10 (C). Representative colonies generated from day 7 (Di–Div) and day 10 (Dv–Dviii) cells are shown, CFU-C activity having been assessed by methylcellulose assays on days 7 and 10 (E). These data represent three independent experiments, and bars indicate the SEM. Flow cytometry analysis to assess the expression of Runx1C-GFP in the CD34/CD43 double-positive cell population; Runx1C-GFP+ cells are shown in green and Runx1C-GFP− cells in red (F). Original magnification ×60. Abbreviations: CFU, colony-forming unit; G, granulocyte; GFP, green fluorescent protein; GM, granulocyte macrophage; M, macrophage; Q, quartile.
Figure 2.
CFU-C activity was enriched within the CD34hiCD43+ cell population. CD34+ cells from day 7 cultures (A) and CD43+ cells from day 10 cultures (C) were sorted using magnetically activated cell sorting enrichment and reanalyzed by flow cytometry to assess purity (unsorted [red], enriched positive cells [blue], and flow-through low/negative cells [green]). Hematopoietic activity was quantified by plating these cell populations in CFU-C assays (B, D). Day 10 cultures were stained for both CD34 and CD43 (E) and CD43+/CD34−, CD43+/CD34+ double-positive, and CD43− CD34− double-negative cells were plated in CFU-C assays to quantify their hematopoietic activity (F). Abbreviations: BFU-E, burst-forming unit erythroid; CFU, colony-forming unit; GM, granulocyte macrophage; M, monocyte; Max, maximum; MIX, mixed; NEG, negative; POS, positive; Q, quartile.
Enforced Expression of HOXB4
HOXB4 has been widely reported to enhance the production of hematopoietic cells from murine ESCs; however, its effect on the differentiation of hESCs is less clear. The variable findings from hESC studies likely result from the variety of differentiation strategies and the different systems used to express HOXB4, resulting in differing expression levels [19]. Based on our previous studies, we hypothesized that activation of exogenous HOXB4 would increase the production of HPC during the differentiation process [20] and might also act to promote a more “definitive-like progenitor” capable of differentiating into mature adult-like RBCs that could express adult globins and enucleate efficiently. To assess the effects of HOXB4 in our defined differentiation protocol, we transfected H1 hESCs with a tamoxifen-inducible HOXB4-ERT2 transgene under the control of the CAG promoter, as previously described [20]. We have previously confirmed and reported that the HOXB4-ERT2 fusion protein can localize to the nucleus with the addition of tamoxifen and that it is functional in this context by demonstrating the induced expression of the HOXB4 target gene, Frzb, with the addition of tamoxifen [20]. The expression of the HOXB4-ERT2 protein and the hematopoietic differentiation potential of hESCs carrying this transgene were confirmed by Western blotting and CFU-C assays (supplemental online Fig. 1).
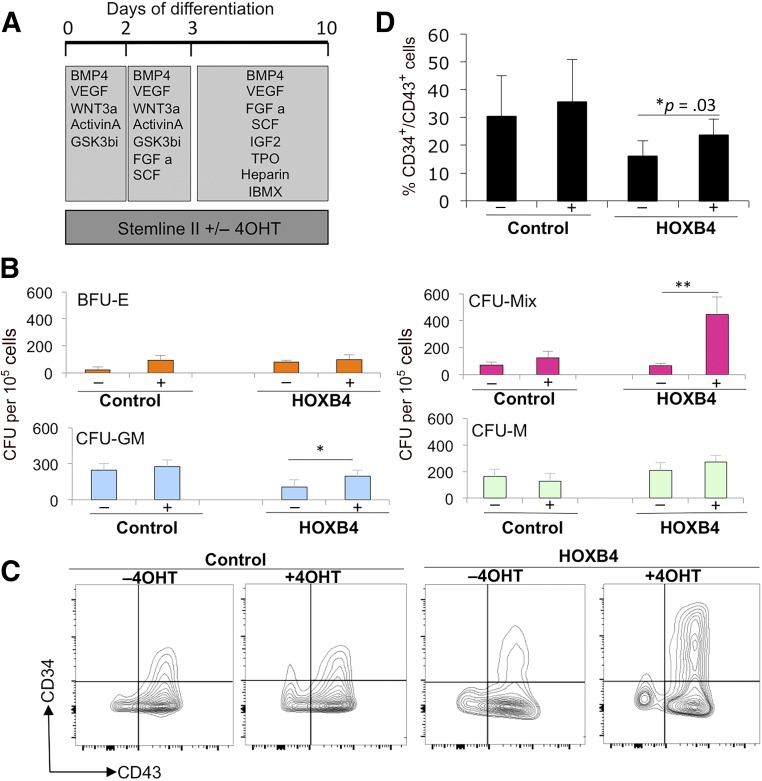
We first tested the effects of HOXB4 activation on the production of HPCs in differentiating hESCs carrying this transgene in the presence and absence of tamoxifen for 10 days by CFU-C assays and flow cytometry (Fig. 3). We observed a significant increase in the number of CFU-Mix and CFU-granulocyte macrophage colonies in the presence of tamoxifen in cells expressing the HOXB4-ERT2 transgene but not in control H1 ESCs (Fig. 3B). No significant difference was found in the number of CFU-macrophage (CFU-M) or burst-forming units-erythroid (BFU-E) in the presence and absence of tamoxifen in either cell line, indicating that enforced expression of HOXB4 preferentially enhanced the production, proliferation, or survival of multipotent HPCs. Consistent with the CFU-C data, the proportion of CD34+CD43+ double-positive cells was increased when HOXB4 was activated by tamoxifen (Fig. 3C, 3D). The overall proportion of cells expressing CD43 did not change when HOXB4 was activated; thus, the increase in double-positive CD34+/CD43+ cells resulted from an increase in the number of cells expressing CD34 (Fig. 3C).
Figure 3.
Activation of HOXB4 enhanced the production of hematopoietic progenitors. Tamoxifen (labeled + or −) was added to cultures to activate HOXB4 in H1 human embryonic stem cells (hESCs) expressing HOXB4-ERT2 (labeled HOXB4) or parental H1 hESCs (labeled control) between days 0 and 10 (A) and then assessed for CFU-C activity (B) and expression of hematopoietic progenitor markers, CD34 and CD43, by flow cytometry (C, D). Data were generated from four independent experiments, with error bars representing SEM. ∗, p = .03; ∗∗, p = .04. Abbreviations: 4OHT, tamoxifen; BFU-E, burst-forming unit erythroid; BMP4, bone morphogenetic protein 4; C, cell; CFU, colony-forming unit; FGF a, fibroblast growth factor-α; GM, granulocyte macrophage; GSK, glycogen synthase kinase; IBMX, isobutylmethylxanthine; IGF2, insulin-like growth factor 2; M, macrophage; Q, quartile; SCF, stem cell factor; TPO, thrombopoietin; VEGF, vascular endothelial growth factor.
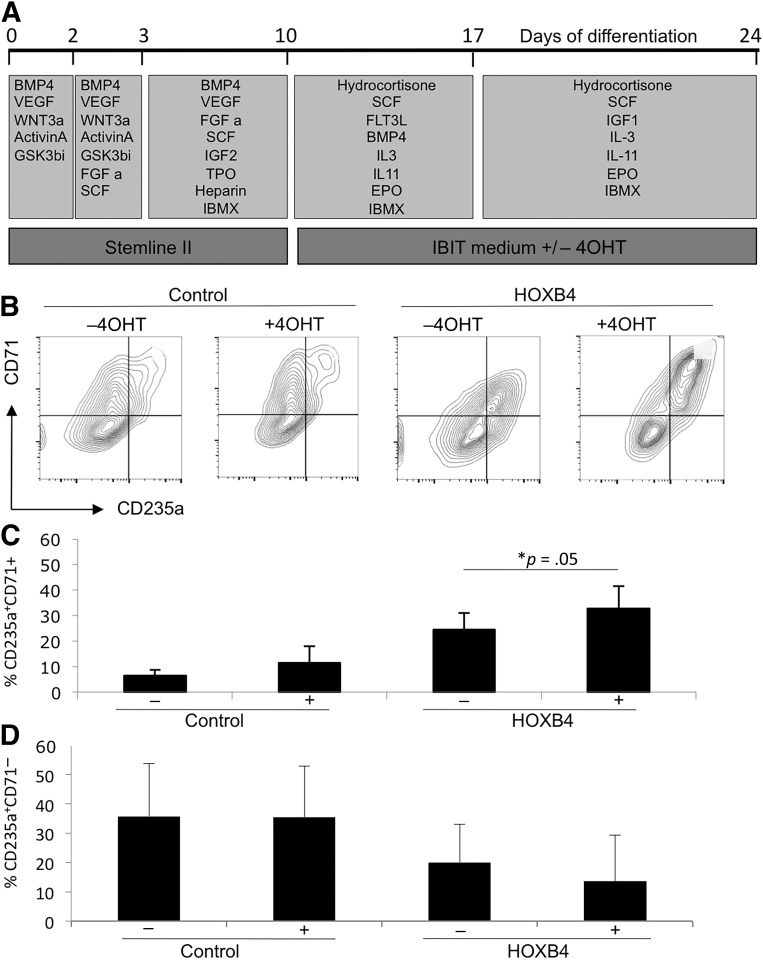
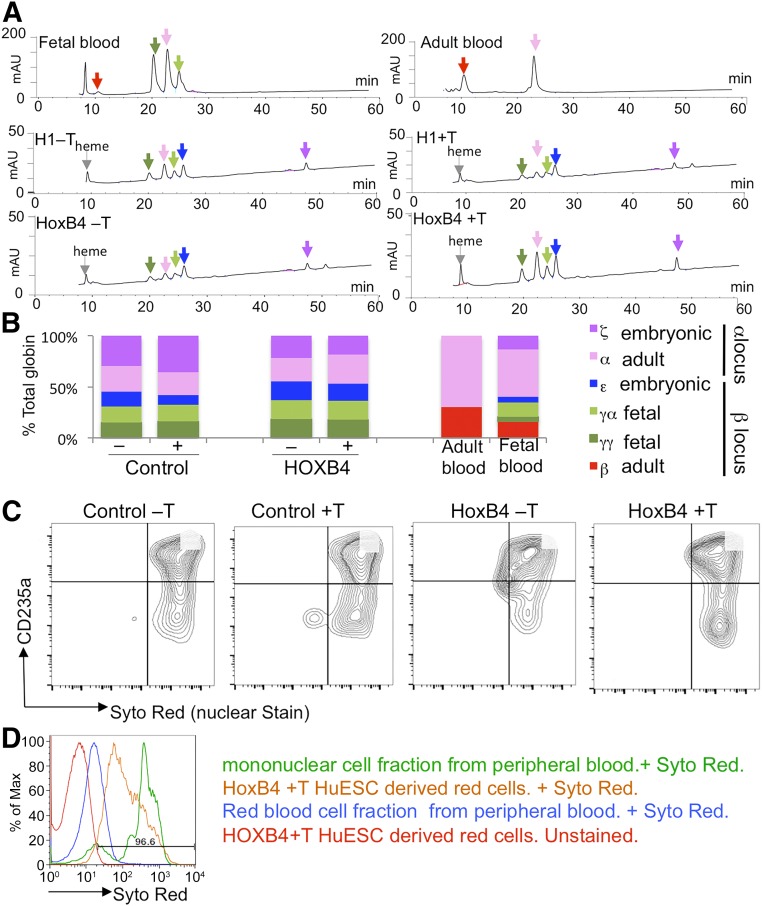
To assess the effects of enforced HOXB4 expression on RBC maturation, we generated HPCs in the absence of tamoxifen and then added tamoxifen to the later stages of differentiation (days 10–24; Fig. 4A). We observed no effect of tamoxifen on the overall proportion of erythroid cells that expressed CD235a at day 24, but we noted differences in the proportion of those CD235a+ cells that coexpressed CD71 (Fig. 4B–4D). The CD71 marker is lost as erythroid cells mature, and we noted that the proportion of the less mature CD235a+/CD71+ cells was significantly increased with HOXB4 activation (Fig. 4C). However, no significant difference was found in the proportion of the more mature cells (Fig. 4D). Hemoglobinized cells were observed in cultures in both the presence and the absence of tamoxifen, and the globin profile of these cell populations was analyzed by HPLC at day 24 (Fig. 5A, 5B). β-globin was detected in control fetal and adult blood samples, but hESC-derived RBCs generated predominantly embryonic (ε) and fetal (γα and γγ) globins. Activation of HOXB4 either from days 10 to 24 (data shown) or from days 0 to 24 (data not shown) did not alter this profile, indicating that it had no effect on maturation of the erythroid cells. We predict that altering the activation time period (e.g., from days 10 to 17 or from days 17 to 24) would have a similar negative effect; however, we could not exclude the possibility that different effects might be observed. We also noted that activation of HOXB4 did not alter the efficiency of enucleation of hESC-derived RBCs as assessed by staining with the SYTO red fluorescent nucleic acid stain (Thermo Fisher Scientific) (Fig. 5C) and compared directly with the white blood cell and red blood cell fractions from peripheral blood (Fig. 5D). Globin profiles and enucleation rates were also analyzed in cultures that were activated with HOXB4 throughout the differentiation protocol (i.e., from day 0 to 24), with similar results (data not shown).
Figure 4.
Activation of HOXB4 resulted in a modest increase in the proportion of immature CD235a+/CD71+ erythroid cells. HOXB4 was activated with 4OHT from days 10 to 24 (A). Production of erythroid cells was monitored by expression of CD235a and CD71 by flow cytometry at day 24 (B–D). Data were generated from four independent experiments with error bars representing the SEM. ∗, p = .05. Abbreviations: 4OHT, tamoxifen; BMP4, bone morphogenetic protein 4; C, cell; CFU, colony-forming unit; EPO, erythropoietin; FGF a, fibroblast growth factor-α; FLT3L, FMS-like tyrosine kinase receptor 3 ligand; GSK, glycogen synthase kinase; IBMX, isobutylmethylxanthine; IGF1, IGF2, insulin-like growth factor 1, 2; IL, interleukin; Q, quartile; SCF, stem cell factor; TPO, thrombopoietin; VEGF, vascular endothelial growth factor.
Figure 5.
HOXB4 activation had no significant effect on the maturation of pluripotent stem cell-derived erythroid cells. High-performance liquid chromatography analysis of cell lysates generated at day 24 of the differentiation protocol from control (H1) and HOXB4-activated cells [in the presence (+T) and absence (−T) of tamoxifen] (A). The amount of the different globins detected as a proportion of the total globin content was calculated in six independent experiments (B). Adult peripheral blood and fetal blood were included for comparison. Enucleation of control and HOXB4-expressing human embryonic stem cells (hESCs) in the presence (+T) and absence (−T) of tamoxifen was assessed by flow cytometry using the nuclear Syto Red stain and the erythroid marker, CD235a (C). A representative histogram of SYTO Red staining of control enucleated peripheral blood samples (blue), nucleated mononuclear cells (green), and hESCs after HOXB4 activation (orange) are shown together with the unstained control hESCs (D). Abbreviation: HuESC, human embryonic stem cell.
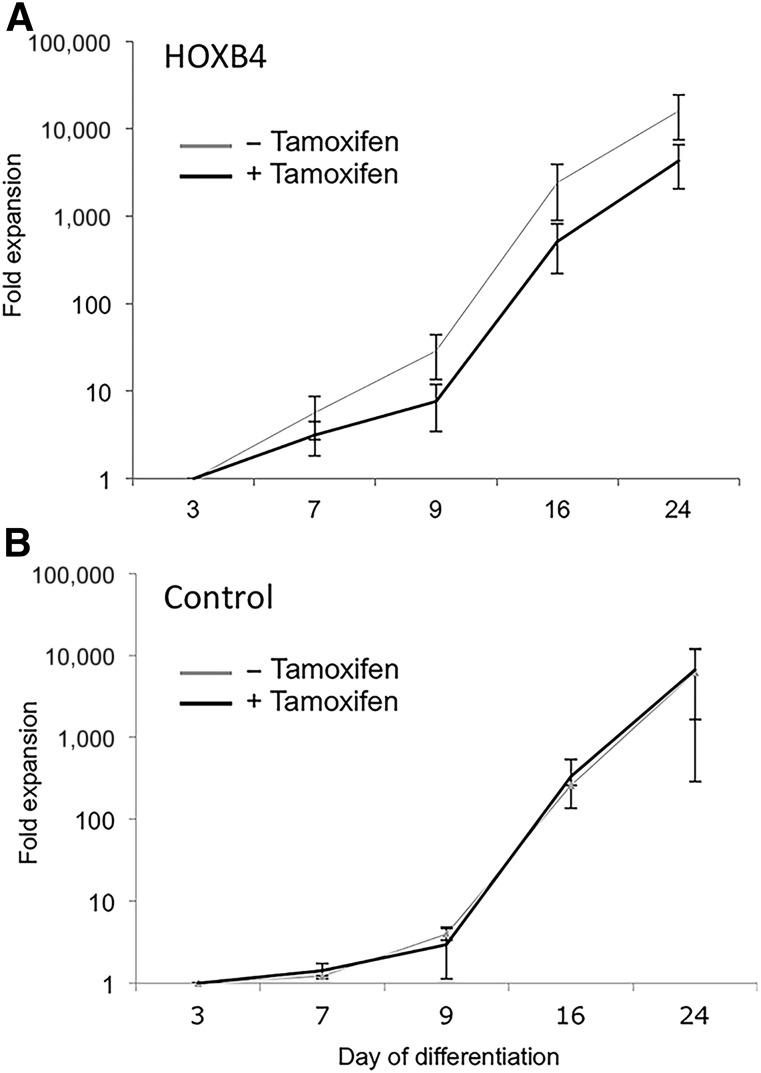
Because one of the aims of the present study was to determine whether HOXB4 activation could enhance the number of RBCs that could be generated, we also assessed the effects of HOXB4 on cell expansion throughout the culture period. A 10,000-fold expansion occurred in cell numbers during the 24-day culture period in the control cells and in the hESCs carrying the HOXB4-ERT2 transgene in the absence of tamoxifen. However, a significant reduction in expansion was observed with HOXB4 activation (Fig. 6). Previous studies have indicated that HOXB4 enhances the proliferation of HPCs ex vivo without significant effects on their differentiation potential [27, 28]. However, we have demonstrated that this cannot be applied to more committed progenitors.
Figure 6.
Cell expansion data for H1 human embryonic stem cells (control) (B) and HOXB4-ERT2-expressing human embryonic stem cells (HOXB4) (A) in the presence (+) and absence (−) of tamoxifen for 24 days. Data are expressed as fold expansion compared with the number of cells at day 3 and represent the mean of six independent experiments (± SE).
Discussion
The development of strategies to produce transfusion-competent RBCs from a limitless supply of human PSCs would have an enormous impact on this widely used therapy. However, to date, most RBCs produced in vitro from this source have had an immature phenotype, characterized by an embryonic or fetal globin profile (or both) and nuclear retention [1]. Although some evidence has shown that iPSC-derived RBCs can undergo terminal differentiation when transplanted in vivo, the nucleated state would preclude the clinical use of these cells [29]. In contrast, RBCs differentiated in vitro from adult, fetal liver, or umbilical cord blood cells enucleated efficiently, demonstrating that it is possible to create a microenvironment in vitro that will support the enucleation process [30, 31]. Adult stem cell-derived enucleated RBCs have been used in a proof-of-principal clinical transfusion study [31]. Although coculture with fetal liver stromal cells can improve the enucleation efficiency of PSC-derived cells, the proportion of enucleated cells remains prohibitively low, and this type of strategy could not be translated into the clinic. A recent study that analyzed the molecular profile of enucleating cells derived from hESCs identified a microRNA (microRNA-30a) as a potential regulator of the enucleation process, demonstrating that it is also possible to modulate the process by altering the intrinsic genetic environment [32].
From our previous findings that enhanced expression of HOXB4 could exert a paracrine effect in differentiating ESCs [20] and that combining coculture with HOXB4 expression did not have an additive effect [21], we sought to test the hypothesis that enhanced expression of this transcription factor could create a microenvironment that would allow for the production of hematopoietic progenitors capable of differentiating into mature erythroid cells. We used a defined, feeder-free culture system designed for the production and expansion of erythroid cells [22].
Production of Hematopoietic Progenitors
We first monitored the production and phenotype of hematopoietic progenitors produced in this defined stepwise protocol. More than 60% of cells expressed HPC markers between days 8 and 10, and the time course of CD43+CD34+ cell production correlated with the production of CFU-C clonogenic progenitors. At this stage, CD43+CD34+ cells predominantly expressed the Runx1C-GFP reporter, supporting their progenitor-like phenotype. The phenotype and potential of PSC-derived hematopoietic progenitors is consistent with that described for differentiating hiPSCs using the OP9 coculture system [33–35]; thus, our robust stepwise protocol is an appropriate system to assess the effects of specific transcription factors on the differentiation process.
Enhanced Expression of HOXB4
We hypothesized that the deficiency in erythroid maturation could result from the primitive nature of hematopoietic progenitors that are generated in vitro. We considered that HOXB4 might be a transcription factor that could program hPSC-derived hematopoietic cells to a more definitive phenotype, because it had been shown to confer long-term reconstitution ability on primitive yolk sac cells and mouse ESCs [16, 17]. The results described in previous studies using human PSCs were contradictory, likely because of differences in the expression strategies and differentiation protocols that were used [19]. To resolve the discrepancies in the published reports, we used a defined differentiation protocol without feeder cells and an inducible expression system in which we could control the timing of the expression of HOXB4. We have demonstrated that enforced expression of HOXB4 increases the production of multilineage hematopoietic progenitors but has no significant effect on the production and maturation of RBCs. The erythroid progenitors that were produced in the presence of HOXB4 failed to undergo efficient enucleation and expressed embryonic (ε) and fetal (γ) but not adult (β) globin. In addition, we observed a detrimental effect of HOXB4 activity on cell expansion, negating any small increases in cell populations seen by inducing HOXB4.
Conclusion
The differentiation system we have defined in the present study generates hematopoietic progenitors and is robust enough to test the effects of the numerous transcription factors shown to program hematopoietic development [36–38]. We have demonstrated that HOXB4 increased the proportion of hematopoietic progenitor production. However, enhanced activity of this transcription factor did not promote red blood cell maturation.
Supplementary Material
Acknowledgments
We thank the following funding agencies: Wellcome Trust, Scottish Funding Council, the Australian Stem Cell Centre, Stem Cells Australia, the National Health and Medical Research Council (NHMRC) of Australia, and the Victorian Government's Operational Infrastructure Support program. A.G.E. and E.G.S. are senior research fellows of the NHMRC. The work was performed as part of the Novosang Consortium (available at http://www.novosang.co.uk).
Author Contributions
M.J.: laboratory research, statistical analysis, manuscript writing; R.M. and R.A.A.: laboratory research, statistical analysis; A.H.T., J.E., M.K., E.O., and L.M.: laboratory research; E.G.S. and J.C.M.: research coordination; A.G.E.: laboratory research, research coordination; L.M.F.: principal investigator, research coordination, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Slukvin II. Hematopoietic specification from human pluripotent stem cells: Current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122:4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lengerke C, Daley GQ. Autologous blood cell therapies from pluripotent stem cells. Blood Rev. 2010;24:27–37. doi: 10.1016/j.blre.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrard T, Bardiaux L, Krause C, et al. Banking of pluripotent adult stem cells as an unlimited source for red blood cell production: Potential applications for alloimmunized patients and rare blood challenges. Transfus Med Rev. 2011;25:206–216. doi: 10.1016/j.tmrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Mountford J, Olivier E, Turner M. Prospects for the manufacture of red cells for transfusion. Br J Haematol. 2010;149:22–34. doi: 10.1111/j.1365-2141.2010.08079.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazurier C, Douay L, Lapillonne H. Red blood cells from induced pluripotent stem cells: Hurdles and developments. Curr Opin Hematol. 2011;18:249–253. doi: 10.1097/MOH.0b013e3283476129. [DOI] [PubMed] [Google Scholar]
- 7.Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebihara Y, Ma F, Tsuji K. Generation of red blood cells from human embryonic/induced pluripotent stem cells for blood transfusion. Int J Hematol. 2012;95:610–616. doi: 10.1007/s12185-012-1107-9. [DOI] [PubMed] [Google Scholar]
- 9.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier EN, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kardel MD, Eaves CJ. Modeling human hematopoietic cell development from pluripotent stem cells. Exp Hematol. 2012;40:601–611. doi: 10.1016/j.exphem.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Chang KH, Huang A, Hirata RK, et al. Globin phenotype of erythroid cells derived from human induced pluripotent stem cells. Blood. 2010;115:2553–2554. doi: 10.1182/blood-2009-11-252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: Advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 16.Kyba M, Perlingeiro RC, Daley GQ. Development of hematopoietic repopulating cells from embryonic stem cells. Methods Enzymol. 2003;365:114–129. doi: 10.1016/s0076-6879(03)65008-1. [DOI] [PubMed] [Google Scholar]
- 17.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 18.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 19.Forrester LM, Jackson M. Mechanism of action of HOXB4 on the hematopoietic differentiation of embryonic stem cells. Stem Cells. 2012;30:379–385. doi: 10.1002/stem.1036. [DOI] [PubMed] [Google Scholar]
- 20.Jackson M, Axton RA, Taylor AH, et al. HOXB4 can enhance the differentiation of embryonic stem cells by modulating the hematopoietic niche. Stem Cells. 2012;30:150–160. doi: 10.1002/stem.782. [DOI] [PubMed] [Google Scholar]
- 21.Gordon-Keylock SA, Jackson M, Huang C, et al. Induction of hematopoietic differentiation of mouse embryonic stem cells by an AGM-derived stromal cell line is not further enhanced by overexpression of HOXB4. Stem Cells Dev. 2010;19:1687–1698. doi: 10.1089/scd.2009.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivier EN, Marenah L, McCahill A et al. High efficiency serum free feeder free erythroid differentiation of human pluripotent stem cells using small molecules. Stem Cells Translational Medicine 2016 (in press). [DOI] [PMC free article] [PubMed]
- 23.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 24.Ditadi A, Sturgeon CM, Tober J, et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat Cell Biol. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- 26.Challen GA, Goodell MA. Runx1 isoforms show differential expression patterns during hematopoietic development but have similar functional effects in adult hematopoietic stem cells. Exp Hematol. 2010;38:403–416. doi: 10.1016/j.exphem.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonchuk J, Sauvageau G, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp Hematol. 2001;29:1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- 28.Amsellem S, Pflumio F, Bardinet D, et al. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med. 2003;9:1423–1427. doi: 10.1038/nm953. [DOI] [PubMed] [Google Scholar]
- 29.Kobari L, Yates F, Oudrhiri N, et al. Human induced pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica. 2012;97:1795–1803. doi: 10.3324/haematol.2011.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miharada K, Nakamura Y. In vitro production of enucleated red blood cells from hematopoietic stem and progenitor cells. Methods Mol Biol. 2012;879:505–512. doi: 10.1007/978-1-61779-815-3_31. [DOI] [PubMed] [Google Scholar]
- 31.Giarratana MC, Rouard H, Dumont A, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouzbeh S, Kobari L, Cambot M, et al. Molecular signature of erythroblast enucleation in human embryonic stem cells. Stem Cells. 2015;33:2431–2441. doi: 10.1002/stem.2027. [DOI] [PubMed] [Google Scholar]
- 33.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi KD, Vodyanik MA, Togarrati PP, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Reports. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riddell J, Gazit R, Garrison BS, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157:549–564. doi: 10.1016/j.cell.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elcheva I, Brok-Volchanskaya V, Kumar A, et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 2014;5:4372. doi: 10.1038/ncomms5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos-Mejía V, Navarro-Montero O, Ayllón V, et al. HOXA9 promotes hematopoietic commitment of human embryonic stem cells. Blood. 2014;124:3065–3075. doi: 10.1182/blood-2014-03-558825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.