Abstract
After cell infection by the human immunodeficiency virus type 1 (HIV-1), nascent viral DNA in the form of a high molecular weight nucleoprotein preintegration complex must be transported to the nucleus of the host cell prior to integration of viral DNA with the host genome. The mechanism used by retroviruses for nuclear targeting of preintegration complexes and dependence on cell division has not been established. Our studies show that, after infection, the preintegration complex of HIV-1 was rapidly transported to the nucleus of the host cell by a process that required ATP but was independent of cell division. Functional HIV-1 integrase, an essential component of the preintegration complex, was not required for nuclear import of these complexes. The ability of HIV-1 to use host cell active transport processes for nuclear import of the viral preintegration complex obviates the requirement for host cell division in establishment of the integrated provirus. These findings are pertinent to our understanding of early events in the life cycle of HIV-1 and to the mode of HIV-1 replication in terminally differentiated nondividing cells such as macrophages (monocytes, tissue macrophages, follicular dendritic cells, and microglial cells).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
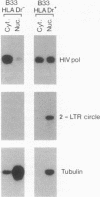
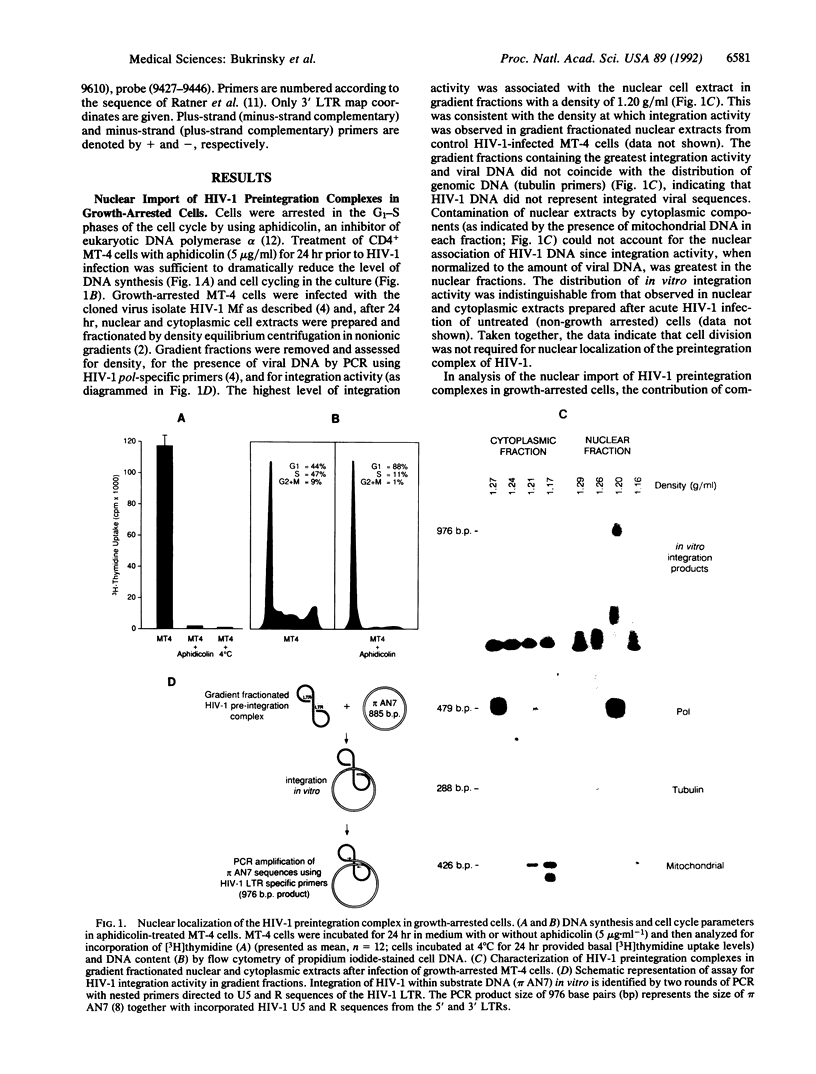
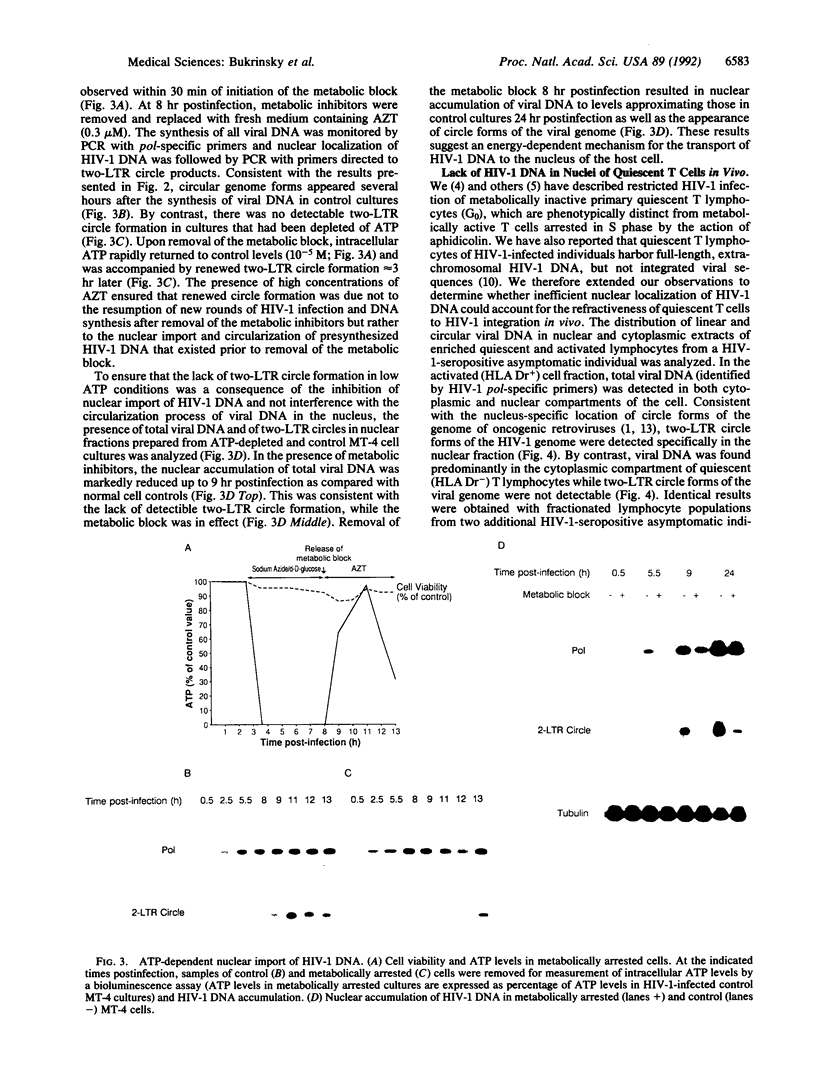
- Bukrinsky M. I., Stanwick T. L., Dempsey M. P., Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991 Oct 18;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison V., Abrams H., Roe T., Lifson J., Brown P. Human immunodeficiency virus integration in a cell-free system. J Virol. 1990 Jun;64(6):2711–2715. doi: 10.1128/jvi.64.6.2711-2715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet C. M., Haseltine W. A. Determination of viral proteins present in the human immunodeficiency virus type 1 preintegration complex. J Virol. 1991 Apr;65(4):1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. T., Hollifield W. C., Seed B., Davie J. M., Huang H. V. Syrinx 2A: an improved lambda phage vector designed for screening DNA libraries by recombination in vivo. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4379–4383. doi: 10.1073/pnas.84.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G. The nuclear and the cytoplasmic pore complex: structure, dynamics, distribution, and evolution. Int Rev Cytol Suppl. 1977;(6):75–186. [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. The terminal nucleotides of retrovirus DNA are required for integration but not virus production. Nature. 1983 Nov 10;306(5939):155–160. doi: 10.1038/306155a0. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S., Miller-Faurès A., Miller A. O., Kruppa J., Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980 Jan 25;8(2):377–387. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Bukrinsky M., Haggerty S. HIV-1 replication and potential targets for intervention. AIDS Res Hum Retroviruses. 1992 Feb;8(2):107–117. doi: 10.1089/aid.1992.8.107. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Haggerty S., Lamonica C. A., Meier C. M., Welch S. K., Wasiak A. J. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J Virol. 1990 May;64(5):2421–2425. doi: 10.1128/jvi.64.5.2421-2425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K., Murakami Y., Miyamoto N., Hanaoka F., Ui M. Assembly of nascent DNA into nucleosome structures in simian virus 40 chromosomes by HeLa cell extract. J Virol. 1990 Oct;64(10):4820–4829. doi: 10.1128/jvi.64.10.4820-4829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Matthews T. J., Cullen B. R., Malim M. H. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J Exp Med. 1991 Dec 1;174(6):1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]