ABSTRACT
This study assessed the effect of endocrine input on the investigation of hyponatraemia and examined the prevalence of endocrine causes of hyponatraemia. This single-centre, retrospective study included 139 inpatients (median age, 74 years) with serum sodium (Na) levels ≤128 mmol/l during hospitalisation at a UK teaching hospital over a three-month period. In total, 61.9% of patients underwent assessment of volume status and 28.8% had paired serum and urine osmolality, and Na measured. In addition, 14.4% of patients received endocrine input; 80% of these patients underwent full work-up of hyponatraemia compared with 5% of patients not referred to endocrine services (p < 0.001; relative risk, 15.86; 95% confidence interval, 7.17–31.06). The prevalence of adrenal insufficiency was 0.7%, but basal serum cortisol levels were not measured in around two-thirds of patients. Despite 26.7% of patients having abnormal thyroid function tests, no patient was diagnosed with severe hypothyroidism. More widespread provision of expert input should be considered.
KEYWORDS: Hyponatraemia, inappropriate ADH syndrome, vasopressin, sodium, investigation
Introduction
Hyponatraemia, the most common electrolyte disorder in hospitalised patients, is associated with considerable morbidity and mortality.1–3 Hyponatraemia can be classified as hypovolaemic, euvolaemic or hypervolaemic, with several possible causes for each category. Treating the underlying aetiology is crucial for management of hyponatraemia; for example, chemotherapy in cases of malignant syndrome of inappropriate antidiuretic hormone secretion (SIADH), or glucocorticoid replacement in cases of adrenal insufficiency. Treatment varies greatly according to the volume status; for example, isotonic saline infusion corrects hypovolaemic hyponatraemia, but is not an effective therapy and may worsen hyponatraemia resulting from SIADH. For these reasons, appropriate investigations and accurate diagnosis are essential for the optimal management of hyponatraemia. Despite this, numerous studies have repeatedly confirmed that hyponatraemia is frequently underinvestigated and mismanaged.4–11
In recent years, numerous studies have highlighted hyponatraemia as an independent risk factor for mortality.1–3 In addition, there is a paucity of data concerning whether investigation of hyponatraemia in clinical practice has improved and which factors determine the adequacy of work-up.
With regard to the endocrine causes of hyponatraemia, besides primary adrenal insufficiency, there has been growing appreciation of secondary adrenal insufficiency as a cause of hyponatraemia;12,13 for example in patients following traumatic brain injury or subarachnoid haemorrhage.14,15 Hyponatraemia due to hypothyroidism is very rare, other than in patients with profound hypothyroidism who meet the criteria for myxoedema coma.16,17 There is a lack of data on the prevalence of endocrine disorders as a cause of inpatient hyponatraemia.
The objectives of this study were to evaluate the adequacy of investigation of hyponatraemia in a tertiary centre, assess the impact of factors, such as expert input, specialty of caring clinical team and serum sodium (sNa) levels, on the adequacy of investigation and determine the proportion of hyponatraemic inpatients being adequately investigated for and diagnosed with adrenal insufficiency and hypothyroidism.
Materials and methods
Study design
This was a retrospective, single-centre study, including all inpatients with sNa levels ≤128 mmol/l at any point during hospitalisation over a three-month period. The study was registered with the Clinical Governance and Clinical Audit Department of our institution.
Patient selection
The study was conducted in a large teaching hospital in the UK with approximately 900 beds. All adult (>18 years old) inpatients, under any specialty, with at least one sNa value ≤128 mmol/l during hospitalisation between 1 March and 31 May 2013, were identified through an automated electronic laboratory system.
A cut-off of 128 mmol/l sNa was selected as previous unpublished data from this hospital cohort showed an upward inflection in inpatient mortality below that threshold, with a mortality rate of 5.8% for 131–132 mmol/l sNa, 8.3% for 129–130 mmol/l sNa, 14.2% for 126–128 mmol/l sNa and 16.9% for ≤125 mmol/l sNa.
Data collection
Hospital case notes, drug prescription charts, discharge letters and laboratory results were reviewed for each patient.
In addition, thyroid function tests, serum cortisol levels and results of short Synacthen tests (serum cortisol levels measured 0, 30 and 60 minutes after intravenous or intramuscular administration of 250 mg cosyntropin) were reviewed. A basal random serum cortisol level >450 nmol/l was considered sufficient to rule out adrenal insufficiency.18,19 The reference range of our laboratory was 12–22 pmol/l for free T4 (fT4) and 0.3–4.2 mU/l for thyroid-stimulating hormone (TSH). Because of the effects of non-thyroidal illness on thyroid function tests in hospitalised patients, the combination of serum TSH levels >20 mU/l with fT4 levels below the reference range was defined as diagnostic of severe primary hypothyroidism.20,21
Statistical analysis
Data were analysed using SPSS (version 21.0; Chicago, IL, USA). Continuous variables were expressed as median (interquartile range (IQR)). Univariate associations between subject groups according to specialty and sNa levels, and categorical variables, such as laboratory investigations, were determined by Chi-squared test. Univariate logistic regression models for the association of the study groups (medical vs surgical patients and ≤125 vs 126–128 mmol/l sNa) with the frequency of performance of various investigations enabled computation of relative risk (RR) with 95% confidence intervals (CIs). p<0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics and outcomes
In total, 139 patients (69 males and 70 females) with a median (IQR) age of 74 (59–82) years developed sNa levels ≤128 mmol/l over the three-month study period. With respect to the timing of onset of hyponatraemia, 43.9% of patients presented on admission with ≤128 mmol/l sNa in comparison to 56.1% who developed hospital-acquired severe hyponatraemia.
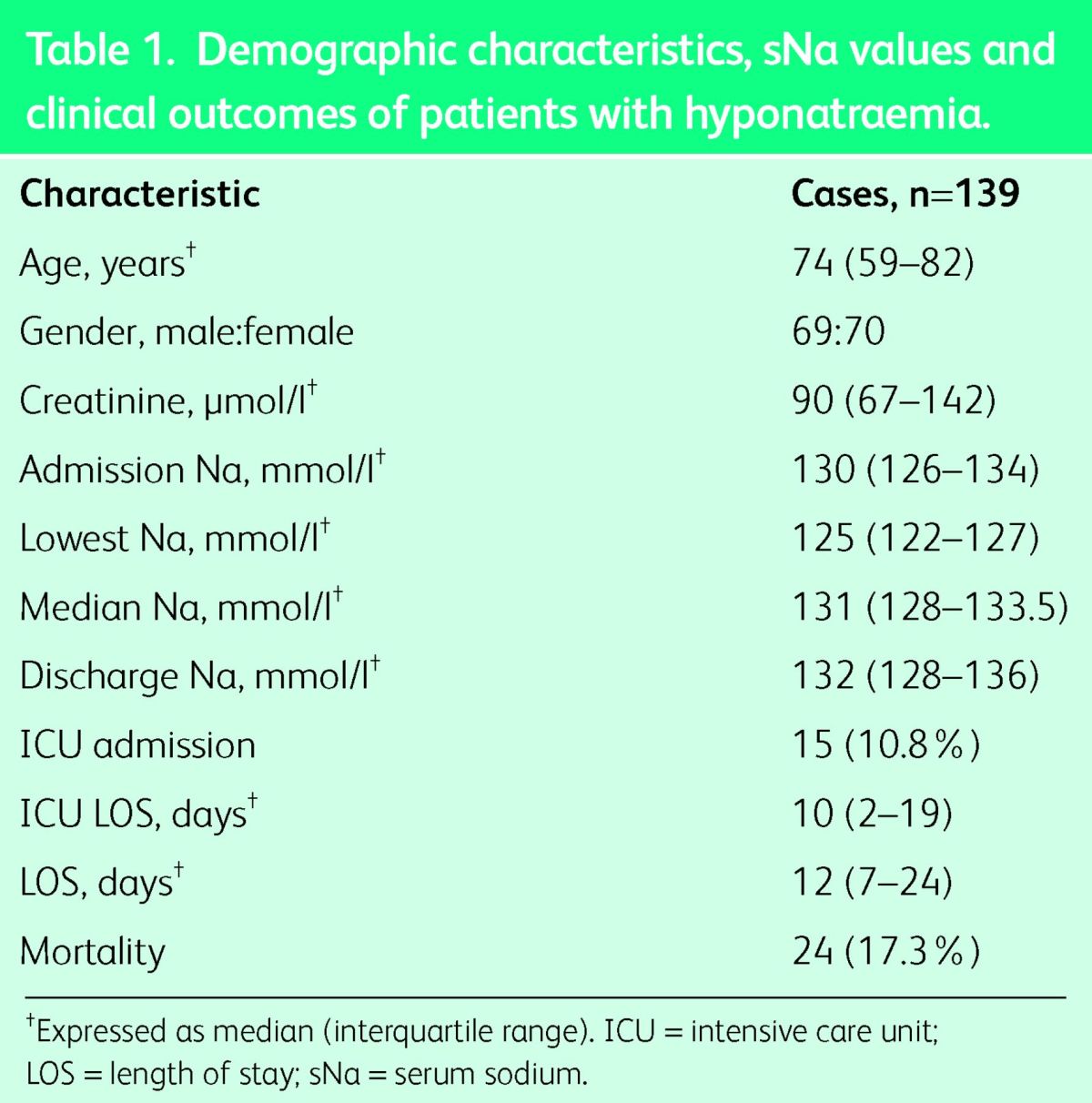
There was a wide distribution of patients within different specialties; 77% of patients were under the care of medical specialties, the most common being geriatrics (16.5%), hepatology (14.4%), general medicine (13.7%), oncology (8.6%), cardiology (5.8%), nephrology (5%) and neurology (5%); 19.4% were under the care of surgical specialties and 3.6% of patients were treated in the intensive care unit (ICU). Our cohort had an in-hospital mortality rate of 17.3% with a median length of hospital stay of 12 days, as shown in Table 1.
Table 1.
Demographic characteristics, sNa values and clinical outcomes of patients with hyponatraemia.
Investigations
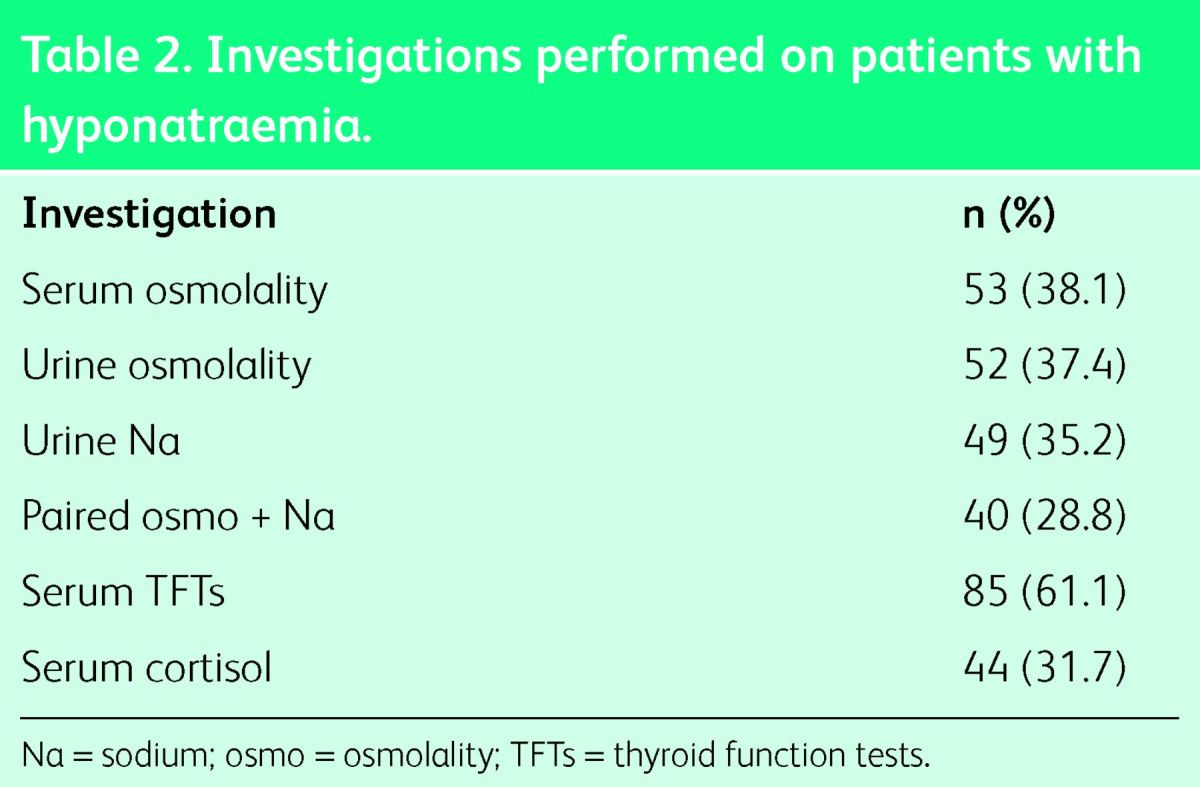
Only 86 patients (61.9%) had their volume status documented in the case notes. The proportion of patients having paired serum and urine osmolality and Na measured was 28.8%, as shown in Table 2.
Table 2.
Investigations performed on patients with hyponatraemia.
Effect of expert input on investigation
Of 139 hyponatraemic inpatients, 20 patients (14.4%) received endocrine input; 80% of them underwent complete clinical and laboratory assessment compared with only 6/119 patients (5%) managed without endocrine input. This difference was statistically significant (p<0.001; RR, 15.86; 95% CI, 7.17–31.06). Complete assessment was defined as documentation of volume status in medical notes, measurement of paired serum and urine osmolality and Na, thyroid function tests and measurement of basal serum cortisol. The median (IQR) time interval between development of ≤128 mmol/l sNa and referral to endocrine services was 4 (1–12) days, with patients requiring, on average, two consultations.
Effect of sNa levels on investigation
With regard to full work-up of hyponatraemia, as defined above, none of the 52 patients with 126–128 mmol/l sNa had the condition compared with 28.7% of patients with ≤125 mmol/l sNa. The effect of sNa value on investigation is summarised in Table 3.
Table 3.
Proportion of patients undergoing investigation according to sNa levels.

Effect of specialty on investigation
Hyponatraemia was not investigated in almost all patients with decompensated cirrhosis under the care of hepatologists or end-stage renal disease under nephrologist care. Besides those patients, there was no statistically significant difference in the proportion of patients having appropriate laboratory investigations between medical and surgical specialties.
Relationship between diagnostic work-up and expert input with outcomes
Patients undergoing complete evaluation had longer hospital stay (median duration, 17 vs 6.5 days), but also lower sNa values (median nadir sNa, 121 vs 125 mmol/l) in comparison to individuals who did not have all tests. Participants who underwent full diagnostic work-up had a mortality rate of 22.7% and ICU admission rate of 18.2 vs 16.2 and 9.4%, respectively, among patients having incomplete evaluation. However, these differences were not statistically significant.
With respect to the relationship between expert input and patient outcomes, patients receiving expert input required longer hospitalisation (median length of hospital stay, 18.5 vs 11 days) and had lower sNa values (median nadir sNa, 119 vs 125 mmol/l) compared with patients without expert input. Individuals receiving expert input had a higher ICU admission rate (30 vs 7.5%; p = 0.009; RR, 3.967; 95% CI, 1.341–10.636) than patients without expert input and a non-statistically significant difference in inpatient mortality (20 vs 16.8%).
Diagnosis
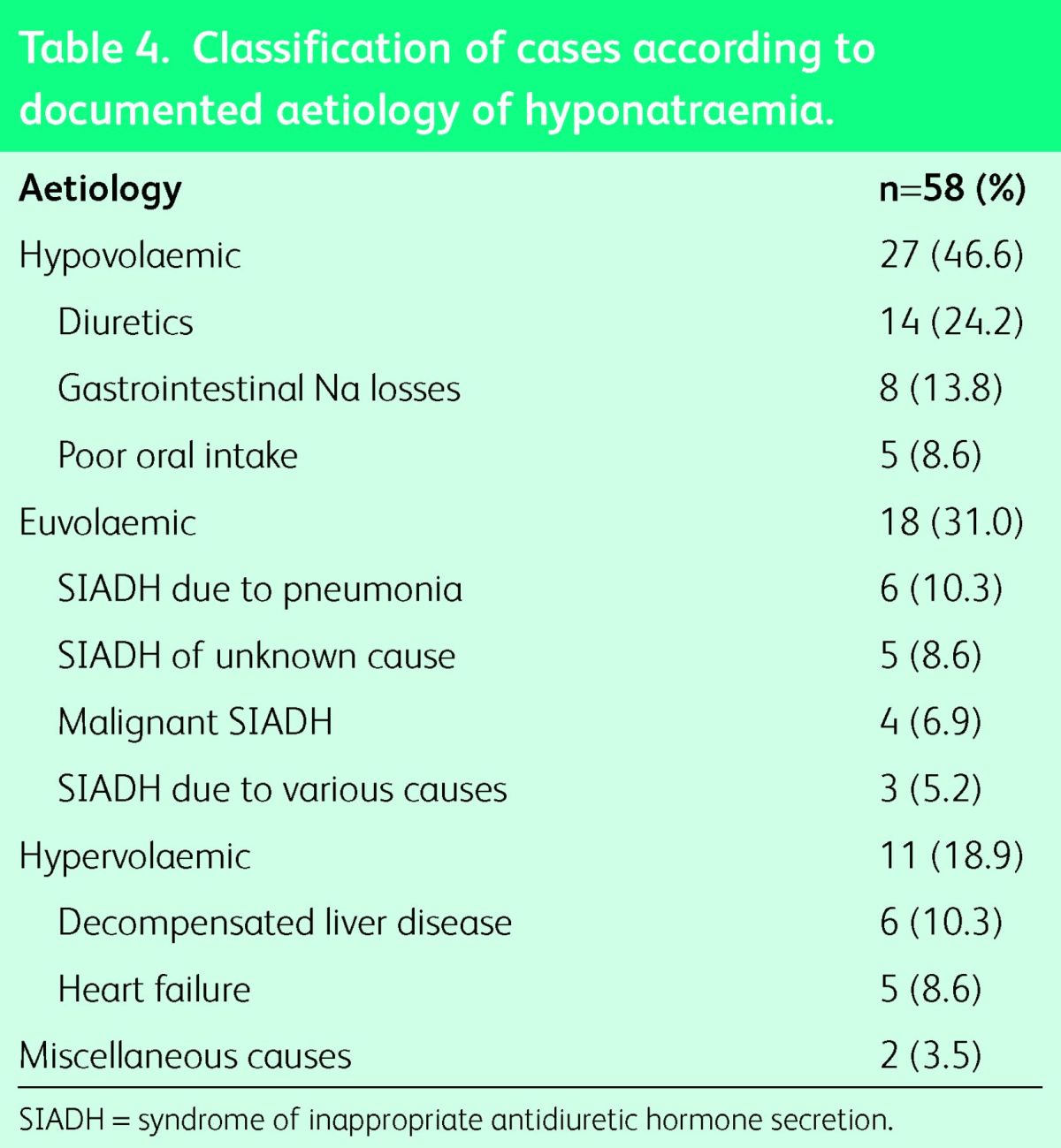
The aetiology of hyponatraemia was recorded in 58 cases (41.7%) only and patients’ ascertainment is accordingly summarised in Table 4. To diagnose SIADH, all of the following criteria must be met: euvolaemia, hyponatraemia and low serum osmolality with inappropriately raised urine osmolality and urine Na, in the presence of normal thyroid and adrenal function.22,23 SIADH was diagnosed in 18 cases, but only 10 patients had all the essential tests performed.
Table 4.
Classification of cases according to documented aetiology of hyponatraemia.
Assessment of glucocorticoid reserve
In our cohort, 13 patients were treated with glucocorticoids for various indications. Among the remaining 126 patients, 44 patients (34.9%) had measurement of basal serum cortisol levels. The median (IQR) basal serum cortisol levels were 584 (442–754) nmol/l with 25% of patients having cortisol levels <450 mmol/l, 36.4% with levels of 450–700 nmol/l and 38.6% with levels >700 nmol/l.
Using a basal serum cortisol of 450 nmol/l as the cut-off point below which glucocorticoid deficiency cannot be excluded,18,24 11 patients justified a short Synacthen test, but this was carried out in 3 patients only. Two patients had an appropriate response, whereas one patient, admitted with septic shock in the ICU, had basal cortisol levels of 337 nmol/l and stimulated levels of 472 nmol/l, prompting a diagnosis of adrenal insufficiency.
Thyroid function tests
fT4 and TSH levels were measured in 85 patients (61.1%) with 73.3% having both fT4 and TSH values within the reference range and the remaining 26.7% with values outside the reference range. The median (IQR) value for fT4 was 16.8 (14.5–19.7) pmol/l (reference range, 12–22 pmol/l) and 2.08 (1.23–3.95) mU/l (reference range, 0.3–4.2 mU/l) for TSH. In addition, 18 patients were on thyroxine replacement.
Among 23 individuals with abnormal thyroid function tests, 9 patients had fT4 values below the reference range (with normal TSH in 3 cases and elevated TSH in 6 cases), 10 patients had normal fT4 levels with raised TSH and 4 patients had fT4 above the reference range with normal TSH. Overall, no patients met the criteria of fT4 below the reference range and TSH levels >20 mU/l, which are strongly indicative of severe primary hypothyroidism in hospitalised patients.
Discussion
Adequacy of investigation
The present study confirmed that hyponatraemia was frequently underinvestigated, although more cases were appropriately investigated than in any other study.4–11 For example, in our hospital cohort, 47.1% of patients with ≤125 mmol/l sNa had urine Na measured and 45.6% had basal serum cortisol measured, compared with 10–18.65,6,8,10 and 8–19%5,7,8,10 respectively in previous studies.
Factors determining adequacy of investigation
This study also demonstrated that review by an endocrinologist and the levels of sNa were the two main factors affecting the adequacy of investigation of hyponatraemia. Patients receiving endocrine input were almost 16 times more likely to have complete clinical and laboratory work-up of hyponatraemia than patients not referred to endocrine services. With respect to sNa levels, clinicians were 4–6 times more likely to perform the basic laboratory investigations for hyponatraemia when the sNa value was ≤125 mmol/l than when the sNa value was 126–128 mmol/l. This observation suggests that some clinicians used 125 mmol/l as a pragmatic, memorable threshold below which they initiated an investigative algorithm.
Adequacy of investigation and patient outcomes
This study did not show a reduced inpatient mortality or length of hospital stay in patients undergoing complete diagnostic work-up or receiving expert input. These findings could suggest that adequate investigation and specialist care provision do not improve patient outcomes or may be misleading due to the distorting effect of significant selection bias. In this study, patients who had full work-up had lower sNa levels and much higher ICU admission rates than patients having inadequate work-up. This observation reflects routine clinical practice when usually patients referred for specialist input represent the most complex cases and the sickest individuals.
Strengths and limitations
This was the first study which examined the association of endocrine input with the adequacy of work-up of hyponatraemia. The main limitations of this study were that it only contained data from a single centre and that the association between endocrine input, level of work-up and outcomes might represent a selection bias with more severe cases being referred to endocrinologists.
Causes of underinvestigation of hyponatraemia
Numerous studies, including this study, have consistently reported inadequate clinical evaluation and underutilisation of biochemical tests. Possible barriers to good clinical practice are the lack of UK guidelines and the absence of diagnostic algorithms in most hospitals or their complexity where they exist. Specifically, the low rate of measurement of serum cortisol may be explained by a lack of awareness of the link between adrenal insufficiency and hyponatraemia and limited confidence in interpreting cortisol levels among doctors.
Specialist input was shown to improve clinical practice and can be provided by various specialties depending on local expertise. In our institution, endocrinologists have developed a special interest in hyponatraemia, while in other hospitals expert input is provided by nephrologists, clinical biochemists, general physicians or geriatricians. Taking into consideration that inpatient hyponatraemia is a common and often complex disorder, which often poses diagnostic challenges even in the hands of the most experienced physicians, an innovative model of care provision by a dedicated hospital ‘hyponatraemia team’ could be developed in major healthcare institutions.
Endocrine causes of hyponatraemia
With regard to adrenal insufficiency, only one case was identified in this cohort of 139 inpatients with hyponatraemia. However, adrenal insufficiency might be overlooked in some cases, since almost three-quarters of the patients did not have laboratory evaluation of their adrenal reserve. Several studies have suggested a threshold of 450 nmol/l for serum cortisol, measured at any time of day, as representing an appropriate glucocorticoid level during acute illness.12,18,19 Using this cut-off, basal cortisol measurement sufficed to exclude adrenal insufficiency in 75% of patients in our cohort. If basal cortisol falls below the cut-off of 450 nmol/l, a short Synacthen test should be performed.18
With respect to hypothyroidism as a cause of hyponatraemia, no cases of severe primary hypothyroidism were identified. However, this study reported abnormal fT4 or TSH values in more than a quarter of hyponatraemic inpatients. These findings are attributed to ‘non-thyroidal illness’ or ‘sick euthyroid syndrome’;21 a syndrome defined by abnormal thyroid function tests in the context of acute illness, despite the absence of an intrinsic abnormality of hypothalamus–pituitary–thyroid function. Therefore, thyroid function tests should be interpreted with great caution since they have very poor specificity in the evaluation of hospitalised patients.20,21
Taking into account that a large proportion of patients were not adequately investigated, this study might underestimate the true prevalence of glucocorticoid deficiency and hypothyroidism among inpatients with hyponatraemia. However, the very small proportion of hyponatraemic patients with endocrine disorders in this cohort raises the question whether all hyponatraemic patients should be screened for adrenal insufficiency and hypothyroidism.
Future studies
Studies are urgently needed to evaluate the clinical- and cost-effectiveness of widespread provision of expert input in cases of hyponatraemia. The use of other innovative tools should also be explored, such as automated ‘biochemical prompting’ systems which alert and prompt clinicians to request appropriate laboratory tests. In addition, prospective studies are warranted to determine whether improvement in diagnostic work-up of hyponatraemia could lead to better patient outcomes.
Conclusions
In conclusion, hyponatraemia remains suboptimally investigated and underdiagnosed; thus, there is an urgent need for further education of healthcare professionals and development of guidelines. It remains to be seen whether development of ‘hyponatraemia teams’, providing more widespread expert input, will improve overall patient outcomes.
References
- 1.Whelan B, Bennett K, O’Riordan D, Silke B. Serum sodium as a risk factor for in-hospital mortality in acute unselected general medical patients. QJM 2009;102:175–82. 10.1093/qjmed/hcn165 [DOI] [PubMed] [Google Scholar]
- 2.Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 2010;170:294–302. 10.1001/archinternmed.2009.513 [DOI] [PubMed] [Google Scholar]
- 3.Corona G, Giuliani C, Parenti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PloS One 2013;8:e80451. 10.1371/journal.pone.0080451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook MA, Velauthar U, Moran L, Griffiths W. Review of investigation and management of severe hyponatraemia in a hospital population. Ann Clin Biochem 1999;36:158–62. 10.1177/000456329903600204 [DOI] [PubMed] [Google Scholar]
- 5.Soran H, Alio Z, Pattison T, et al. Management of hyponatraemia: are we doing enough? QJM 2005;98:620–1. 10.1093/qjmed/hci100 [DOI] [PubMed] [Google Scholar]
- 6.Saeed BO, Beaumont D, Handley GH, Weaver JU. Severe hyponatraemia: investigation and management in a district general hospital. J Clin Pathol 2002;55:893–6. 10.1136/jcp.55.12.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton JA, Le Jeune IR, Hall IP. Severe hyponatraemia in medical -in-patients: aetiology, assessment and outcome. QJM 2006;99:505–11. 10.1093/qjmed/hcl071 [DOI] [PubMed] [Google Scholar]
- 8.Huda MS, Boyd A, Skagen K, et al. Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J 2006;82:216–9. 10.1136/pmj.2005.036947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyte M, Down C, Miell J, Crook M. Lack of laboratory assessment of severe hyponatraemia is associated with detrimental clinical outcomes in hospitalised patients. Int J Clin Prac 2009;63:1451–5. 10.1111/j.1742-1241.2009.02037.x [DOI] [PubMed] [Google Scholar]
- 10.Siddique H, Kahal H, Tahrani AA, et al. The management of hyponatraemia at two district general hospitals in the UK. J Eval Clin Prac 2010;16:1353–6. 10.1111/j.1365-2753.2009.01252.x [DOI] [PubMed] [Google Scholar]
- 11.Olsson K, Ohlin B, Melander O. Epidemiology and characteristics of hyponatremia in the emergency department. Eur J Intern Med 2013;24:110–6. 10.1016/j.ejim.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 12.Diederich S, Franzen NF, Bahr V, Oelkers W. Severe hyponatremia due to hypopituitarism with adrenal insufficiency: report on 28 cases. Eur J Endocrin 2003;148:609–17. 10.1530/eje.0.1480609 [DOI] [PubMed] [Google Scholar]
- 13.Liamis G, Milionis HJ, Elisaf M. Endocrine disorders: causes of hyponatremia not to neglect. Ann Med 2011;43:179–87. 10.3109/07853890.2010.530680 [DOI] [PubMed] [Google Scholar]
- 14.Agha A, Sherlock M, Thompson CJ. Post-traumatic hyponatraemia due to acute hypopituitarism. QJM 2005;98:463–4. 10.1093/qjmed/hci075 [DOI] [PubMed] [Google Scholar]
- 15.Hannon MJ, Behan LA, O’Brien MM, et al. Hyponatremia following mild/moderate subarachnoid hemorrhage is due to SIAD and glucocorticoid deficiency and not cerebral salt wasting. J Clin Endo Metab 2014;99:291–8. 10.1210/jc.2013-3032 [DOI] [PubMed] [Google Scholar]
- 16.Warner MH, Holding S, Kilpatrick ES. The effect of newly diagnosed hypothyroidism on serum sodium concentrations: a retrospective study. Clin Endocrinol (Oxf) 2006;64:598–9. 10.1111/j.1365-2265.2006.02489.x [DOI] [PubMed] [Google Scholar]
- 17.Schwarz C, Leichtle AB, Arampatzis S, et al. Thyroid function and serum electrolytes: does an association really exist? Swiss Med Wkly 2012;142:w13669. [DOI] [PubMed] [Google Scholar]
- 18.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003;348:727–34. 10.1056/NEJMra020529 [DOI] [PubMed] [Google Scholar]
- 19.Hagg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 1987;26:221–6. 10.1111/j.1365-2265.1987.tb00780.x [DOI] [PubMed] [Google Scholar]
- 20.Spencer CA, LoPresti JS, Patel A, et al. Applications of a new -chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endo Metab 1990;70:453–60. 10.1210/jcem-70-2-453 [DOI] [PubMed] [Google Scholar]
- 21.Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of -thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987;33:1391–6. [PubMed] [Google Scholar]
- 22.Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med 1967;42:790–806. 10.1016/0002-9343(67)90096-4 [DOI] [PubMed] [Google Scholar]
- 23.Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064–72. 10.1056/NEJMcp066837 [DOI] [PubMed] [Google Scholar]
- 24.Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med 2003;139:194–204. 10.7326/0003-4819-139-3-200308050-00017 [DOI] [PubMed] [Google Scholar]


