Abstract
Where once global health decisions were largely the domain of national governments and the World Health Organization, today networks of international organizations, governments, private philanthropies and other entities are actively shaping public policy. However, there is still limited understanding of how global networks form, how they create institutions, how they promote and sustain collective action, and how they adapt to changes in the policy environment. Understanding these processes is crucial to understanding their effectiveness: whether and how global networks influence policy and public health outcomes. This study seeks to address these gaps through the examination of the global network to stop tuberculosis (TB) and the factors influencing its effectiveness over time. Drawing from ∼200 document sources and 16 interviews with key informants, we trace the development of the Global Partnership to Stop TB and its work over the past decade. We find that having a centralized core group and a strategic brand helped the network to coalesce around a primary intervention strategy, directly observed treatment short course. This strategy was created before the network was formalized, and helped bring in donors, ministries of health and other organizations committed to fighting TB—growing the network. Adaptations to this strategy, the creation of a consensus-based Global Plan, and the creation of a variety of participatory venues for discussion, helped to expand and sustain the network. Presently, however, tensions have become more apparent within the network as it struggles with changing internal political dynamics and the evolution of the disease. While centralization and stability helped to launch and grow the network, the institutionalization of governance and strategy may have constrained adaptation. Institutionalization and centralization may, therefore, facilitate short-term success for networks, but may end up complicating longer-term effectiveness.
Keywords: Global health policy, governance, networks, policy adoption, policy analysis, tuberculosis
KEY MESSAGES.
The re-emergence of tuberculosis (TB) as a global emergency, the leadership of the World Health Organization (WHO), a central intervention strategy [directly observed treatment short course (DOTS)], and a supportive policy environment contributed to the emergence of the TB network
The TB network was generally perceived to have been effective in gaining attention and resources for TB, as well as advancing DOTS in high burden countries; however, over time, it has faced the challenges of a changing epidemic and internal tensions surrounding network governance and priorities
Institutionalization of governance and strategy helped facilitate short-term success for the network by supporting scalability, the ability of the network to grow rapidly at low cost, but it may also be complicating longer-term effectiveness by hampering adaptation
Therefore, the processes of scalability and adaptability may be in conflict with one another, posing a significant challenge for sustaining network effectiveness over time
Introduction
Where once global health decisions were largely the domain of national governments and the World Health Organization (WHO), today networks of international organizations, governments, private philanthropies and other entities are actively shaping public policy (Walt 2004). It has been proposed that the rise, persistence and decline of a health issue may best be explained by the way in which its global network—the system of relations between individuals and organizations working in concert to address a complex problem—comes to understand and portray the issue, as well as its ability to establish institutions that can sustain this portrayal (Shiffman 2009). However, there is still limited understanding of how global networks form, how they create institutions, how they promote and sustain collective action and how they adapt to changes in the policy environment (Kahler 2009). Understanding these processes is crucial to understanding their effectiveness: whether and how global networks influence policy and public health outcomes. This study seeks to address these gaps through the examination of the global network to stop tuberculosis (TB) and the factors influencing its effectiveness over time.
TB has been present in humans for centuries, yet as with many global health problems, it remains a public health paradox: in spite of known preventive and curative interventions, it continues to be a major global cause of mortality and morbidity (Porter and Grange 1999). In 2012, there were 8.6 million incident cases of TB and 1.3 million deaths (WHO 2013). An estimated 11–13% of incident cases and approximately one-third of deaths were in HIV-positive patients (WHO 2012b). Treatment of TB is also complex. Diagnosis is sometimes difficult, and anti-TB drugs have to be taken over a relatively long period (Lawn and Zumla 2011). TB programs have become increasingly complicated with the rapid growth in detection and reporting of multi-drug resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB; Reingold and Gordon 2012).
Although TB affects both wealthy and poor, it is primarily a disease of poverty. Over 95% of prevalent cases and deaths from TB occur in lower and middle-income countries (WHO 2010). Within countries poverty is also associated with increased risk of TB infection. For example, in India, prevalence rates are about five times higher in the poorest quintile of the population than in the wealthiest quintile (Oxlade and Murray 2012). Over-crowding increases the risk of airborne transmission, and weakened immune systems (e.g. from poor nutrition or HIV) are particularly vulnerable to TB (WHO 2010). Poverty also decreases the chances that people will receive treatment, thus spreading infections (Reingold and Gordon 2012). Without treatment, about 70% of people with active pulmonary TB will die in under 10 years (WHO 2012b).
In the late 1980s, crucial events raised attention for TB: the rise in TB incidence in high-income countries, the scientific link between TB and HIV, and the increase in multi-drug resistance to treatment (Raviglione 2003). Over the next decade, a loosely formed network of organizations, led by individuals at WHO, sought greater resources and policy attention for TB. This network officially coalesced in 2001 as the Global Partnership to Stop TB, and is broadly considered to have played a key role in promoting TB control, treatment and research through its development of the central global TB control strategy directly observed treatment short course (DOTS), its mobilization of widespread political commitment to the Global Plan to Stop TB, and its engagement with funding partners such as USAID and the Global Fund to Fight AIDS, TB and Malaria, which led to increased financial support for TB (Caines et al. 2003; Buse and Harmer 2007; McKinsey & Co. 2008; Buse and Tanaka 2011). The network’s membership has expanded significantly over time, DOTS has been adopted in over 180 countries, and it is estimated that 22 million lives have been saved compared to the previous standard of care (WHO 2013). In this article, we explore how this network emerged, developed institutions and responded to changing circumstances, as well as how these processes have influenced network effectiveness over the past decade.
Conceptual framework
This study is part of the Global Health Advocacy and Policy Project (GHAPP), a research initiative examining networks that have mobilized to address six global health problems—maternal mortality, neonatal mortality, TB, pneumonia, tobacco use and alcohol harm. The aim of GHAPP is to understand why networks coalesce more easily for some issues but not others, and why some are more effective at influencing policy and public health outcomes. Individual cases seek to identify key factors shaping that particular network’s emergence and effectiveness, with the concluding paper to the supplement comparing and synthesizing these factors across the six cases (see Shiffman et al., 2016).
Network effectiveness in this project is defined as: the extent to which networks are able to change the world to meet their member’s perceptions of what reality should look like (Woolcock and Narayan 2000; Sikkink 2009). We examine network effectiveness by considering outputs, policy consequences and impact. Outputs are the immediate products of network activity, such as the network’s capacity to attract attention and develop interventions. Policy consequences pertain to the global policy process, including international resolutions, funding and convincing national governments to adopt policies and carry out programmes. Impact refers to whether or not these activities contribute to an improvement in population health. Additionally, we consider how the activities and organizing structures influence both short-term effectiveness, such as initial attempts at getting TB onto the global agenda and encouraging policy adoption at the national level, and long-term effectiveness. Key aspects of long-term effectiveness include: the continued mobilization of resources, the adaptation of strategies and processes to changes in network membership and in the epidemiology of the disease and the ability to sustain and improve cure rates.
GHAPP studies draw on a common conceptual framework grounded in theory on collective action from political science, sociology and economics (Kingdon 1984; Snow et al. 1986; Stone 1989; Powell 1990; Finnemore and Sikkink 1998; Keck and Sikkink 1998; Marsh and Smith 2000; McAdam et al. 2001; Kahler 2009). The introductory article to this supplement presents the framework in detail (see Shiffman et al., 2016). The framework consists of three categories of factors. One category, network and actor features, concerns factors internal to the network involving strategy and structure and attributes of the actors that constitute the network or are involved in creating it. This category pertains to how networks and the individuals and organizations that create and comprise them exercise agency. A second category, the policy environment, concerns factors external to the network that shape the network itself and the effects the network hopes to produce. The third category, issue characteristics, concerns features of the problem the network seeks to address. The idea is that issues vary on a number of dimensions that make them more or less difficult to tackle. GHAPP studies begin with the presumption that no single category of factors is determinative: rather factors in each of the three interact with one another to shape policy and public health effects.
In each category, there are several factors that may be particularly influential. Among network and actor features, the existence of effective ‘leaders’ (factor 1) may be one reason networks coalesce in the first place, and why, once they appear, they are able to achieve their objectives. The system of ‘governance’ (factor 2) may also matter: the effectiveness of the institutions network members set up to steer themselves towards collective goals (Buse and Walt 2000). Three primary modes of network governance have been identified: (1) shared, where most or all network members interact on a relatively equal basis to make decisions; (2) lead organization, where all major network-level activities and key decisions are co-ordinated through and by a single participating member and (3) network administrative organization, where a separate entity is set up specifically to govern the network and its activities (Provan and Kenis 2008). One form of governance is not necessarily better than others, but it must be responsive to characteristics of the network, the issue and the policy environment. A third factor is ‘composition’ (factor 3). Diverse networks that link scientists, advocates, policy-makers and others from both high- and low-income countries may achieve better outcomes than uniform ones because diversity improves collective understanding and problem solving, among other benefits (Hong and Page 2004; Page 2008). However, diversity may also lead to greater goal conflict within the network. The fourth factor is ‘framing strategy’ (factor 4; Snow et al. 1986; McInnes et al. 2012): how network actors publicly position an issue in order to attract attention and resources. Networks may differ in how they frame their issues, potentially contributing to differences in network effectiveness. Additionally, there may be internal disagreement about how to define the problem and the most appropriate solution. How networks manage conflict over strategy will also have implications for the sustainability of network activities.
Several factors in the policy environment may be particularly influential. Among these are ‘potential allies and opponents’ (factor 5). If there are many groups whose interests align with a network’s goals, that network is more likely to expand and be effective than one that faces a dearth of potential allies. Opponents may both hinder and facilitate network outcomes: they may seek to discredit the network, but may also inspire mobilization. Substantial ‘funding’ (factor 6) may enable a network to flourish; however, a network set up at the behest of donors may be perceived as less legitimate than those that emerge from grassroots activism. ‘Norms’ (factor 7)—standards of appropriate behaviour for a particular group of actors—may also be influential (Katzenstein 1996; Finnemore and Sikkink 1998). The starkest examples of influential norms in global health are those that the health-related Millennium Development Goals (MDGs) advance (Fukuda-Parr and Hulme 2011). These goals have raised expectations that states, international organizations and other global actors act to reduce the burden of the global health problems selected for inclusion.
Among issue characteristics, ‘severity’ (factor 8), ‘tractability’ (factor 9) and the nature of ‘affected groups’ (factor 10) may be particularly influential. Robust networks may be more likely to emerge when problems lead to high mortality and morbidity or social disruption—or are perceived to do so. Also, individuals and organizations may be more likely to act on problems perceived to be soluble (Stone 1989). In addition, affected populations that inspire sympathy, such as children, may be more likely to inspire network mobilization (Stone 1989; Schneider and Ingram 1993) than those that do not.
From these framework factors, this study pays particular attention to network and actor attributes in the emergence and effectiveness of the global TB network. Additionally, we consider several network attributes that are more relevant for older, well-established networks: institutionalization, scalability and adaptability. The interaction between these factors, issue characteristics and the policy environment are examined historically, but with an emphasis on 2001 until present.
As networks form, the decisions surrounding composition, governance and strategy may establish rules and norms that will continue to structure interactions between network members, leading to the ‘institutionalization’ of these initial choices (Ostrom 2007). In other words, once a network starts down a particular track, previous decisions shape the possibilities for future decisions, and the costs of reversing the initial choices may increase over time, leading to path dependency (Pierson 2000). Path dependency can become problematic if changes in the network itself, in the policy environment, or in the issue with which the network is concerned, require a shift in course. How networks alter initial choices in response to changing conditions has received limited attention in the literature as most network studies only consider networks at one point in time. This study draws attention to the process of institutionalization, and examines how early decisions shaped the ability of the TB network to expand its reach and to adapt over time, two subsequent processes that are thought to influence network effectiveness.
Existing scholarship proposes that successful networks will exhibit scalability and adaptability. ‘Scalability’ refers to the ability of networks to grow rapidly at relatively low cost (Kahler 2009). To do so, a certain amount of structural stability is necessary to ensure that a network can expand its reach quickly without fracturing or disintegrating. ‘Adaptability’ indicates that a network is able to change the rules, norms and strategies structuring networks as political demands shift or the environment changes (Kahler 2009). What is left un-discussed is the potential tension between these two processes. Institutionalization may support scalability, making it more difficult to change rules, norms and strategies as the network ages. Adapting rules, norms and strategies to changing circumstances may be necessary for sustaining collective action and the ability of networks to influence policy and health outcomes over time.
Methods
This study used a process-tracing methodology involving in-depth examination of social and political processes in order to uncover causal mechanisms that led to the policy and public health outcomes being investigated (Yin 2008; Bennett 2010). The aim was to trace in detail the role of networks, environments, issue characteristics and other factors in shaping agenda-setting, policy formulation, policy implementation and mortality and morbidity change. GHAPP researchers used the same methodology, began with the same basic set of questions and were in frequent communication in order to share insights as the studies unfolded. It is also worth mentioning that detecting network influence is more difficult as one moves from outputs to policy consequences to impact. It may be more straightforward to demonstrate that TB network members developed an intervention strategy, proposed a specific policy or helped to secure funding. Determining the role of these activities in improving TB mortality is considerably more complex.
As the focus of this study, and the entire GHAPP project, is on how global networks form, evolve and seek to influence national policies, priority is given to global-level data. To minimize bias, the study drew on multiple sources of information. Documents comprised the primary data source. Approximately 200 published and unpublished documents were analysed from donors, governments, international organizations, scientific journals and media outlets, including several which provided historical analyses of the Stop TB Partnership (Porter and Grange 1999; Raviglione and Pio 2002; Raviglione 2003; Espinal 2012; Raviglione et al. 2012). We also drew extensively from aggregate national data found in 13 years of Global TB Reports and numerous cross-national studies published in academic journals (Walt et al. 2004; Ramon-Pardo et al. 2008; Dye et al. 2009, Lönnroth et al. 2009; Nair et al. 2010; WHO 1998–2013). These documents were identified using PubMed, Google Scholar, JSTOR and ProQuest searches. Document sources were used to identify key people and events, to provide information on the content of policies and advocacy efforts, and as means of assessing the effectiveness of the TB Partnership. For example, previous evaluations of the Stop TB Partnership were used as one metric of network effectiveness, primarily on the effectiveness of network processes and outputs, for the time period in which they were published (Caines et al. 2003; McKinsey & Co. 2008). Cross-national data were used to assess policy consequences and impact of network recommended policies.
We supplemented our document sources with targeted key informant interviews to gather different perspectives on the effectiveness and evolution of the network. The 16 individuals interviewed were purposively selected because of their role, or the role of their organizations, as central actors in the creation and evolution of the network. The organizations include: The Bill and Melinda Gates Foundation; The Centers for Disease Control and Prevention; The Foundation for Innovative New Diagnostics; The Global Fund to Fight AIDS, TB and Malaria; The International Union for Tuberculosis and Lung Disease; The London School of Hygiene and Tropical Medicine; Partners in Health; The Stop TB Partnership Secretariat; The Stop TB Department, WHO; Treatment Action Group; The World Bank and The WHO. The individuals selected from these organizations were identified from the literature search and were chosen because they have a significant depth of historical knowledge, they have been deeply involved in the activities of the network, they represent the main sub-groups of the network working at the global level (WHO, the Partnership Secretariat, donor organizations, research and development organizations, academics and non-governmental organization [NGO] activists), and many have significant experience with national-level TB programmes. Interviews were conducted in person and over the phone, each lasting between 1 and 2 h. When possible and with permission, they were recorded and transcribed. Interviews and documents were manually coded according to the conceptual framework, using a coding scheme developed and shared across all six GHAPP cases. Finally, drafts of the case study were reviewed by three members of the GHAPP team, one academic expert in the TB field, four members of the policy network and an academic expert in global health policy.
The primary limitation of this study is that we did not gather a breadth of responses across the large network. Time and logistic constraints prevented us from interviewing health ministries of high TB burden countries, representatives from National TB Programs, or members of domestic civil society groups, making assessments of national-level perceptions difficult. We acknowledge this as a shortcoming, given that global policies are ultimately implemented at the country level. To address this issue, we opted for an assessment of national policy data to draw inferences on network effectiveness. For example, policy data show when countries first adopted DOTS programmes and the extent to which these programmes cover affected populations. As will be discussed in more detail, DOTS policies originated at WHO and were heavily promoted first by WHO and later by the TB network. Tracking the adoption and scale-up of DOTS using data reported to the network as well as national-level studies of TB policy provides evidence of global influence on national policies over time. What is more challenging is connecting policies to health outcomes. We use data on cure rates and information from published papers on policy effectiveness to analyse this issue in more detail.
Results
The emergence of the global TB network
An international network to address TB dates back to 1867 (IUATLD 2010), but changes in attention to the disease over the mid-1900s altered the number of organizations and individuals working collaboratively. While TB was a leading cause of death from the 1600s to the early 1900s, improved living conditions and then the advent of antibiotic treatment in the 1950s led to an accelerated decline in mortality and morbidity in the industrialized world. As a result, attention to TB diminished, and the period from the 1960s to the late 1980s has been characterized as one of complacency (Walt 1999). Declines in TB mortality contributed to perceptions that TB could be eliminated in high-income countries, while TB Control Programs in low-income countries would address problems in those parts of the world (Raviglione and Pio 2002). A narrow scientific community continued researching strategies for treatment, but decreasing urgency in Europe and the United States contributed to decreasing activity at the global level.
The emergence of HIV and MDR-TB, particularly in high-income countries, can be viewed as the focusing event that redefined TB as a public health problem (Ogden et al. 2003), and from the late 1980s, global attention to TB began to re-emerge. The perception that the severity of the disease was escalating contributed to a sense of crisis, and one of the central organizations to respond was the WHO. In 1993, WHO declared TB a global emergency (Ogden et al. 2003). That same year, the World Bank’s Development Report ‘Investing in Health’ published findings from studies on the cost-effectiveness of health interventions. Short course chemotherapy for TB was found to be ‘one of the most cost-effective of all interventions’ (World Bank 1993, p. 63). A year later, WHO’s ‘Framework for Effective TB Control’ was developed (Lee and Buse 2002; Ogden et al. 2003). Packaged as ‘DOTS’, with DOT standing for ‘directly observed treatment’ and the S for ‘short course’, it became an attractive, simple message to the world that TB could, should and would be contained—giving the problem tractability. By the mid-1990s, the DOTS policy had been adopted in 20 countries (WHO 1995). The whole DOTS strategy included five components: (1) political commitment with increased and sustained financing, (2) case detection through quality-assured bacteriology, (3) standardized treatment with supervision and patient support, (4) an effective drug supply and management system and (5) monitoring and evaluation for impact measurement. While it was a more comprehensive approach than the acronym suggested, the DOTS approach was frequently interpreted in its more narrow sense of directly observed TB treatment.
This branding of DOTS was not universally accepted within the TB research community, contributing to difficulties in collaborative action. Conflicting views were expressed by those experts who supported DOTS and other technical and scientific experts who were concerned that the new strategy would mean less funding for research and development, that the emphasis on directly observed treatment was operationally and ethically problematic, and that DOTS oversimplified an extremely complex problem (Editorial 1994; Zwarenstein et al. 1998; Ogden et al. 2003). However, in spite of these differences of opinion, WHO recognized that the DOTS ‘message’ resonated well with donors and with ministries of health who saw the strategy as an easy way of communicating what needed to be done (Interview 5). DOTS became the foundation of global TB policy advocacy, with WHO leading the charge.
Those working in TB at WHO formed the Global TB Program in 1995, which aggressively marketed the DOTS strategy (Lee and Buse 2002), and established the TB Global Surveillance and Monitoring System to collect data on disease prevalence (including MDR-TB), and the scale-up of DOTS (Pablos-Méndez et al. 1998). By 1997, the global status of TB control and the progress towards achieving WHO targets of curing 85% of sputum smear-positive patients and detecting 70% of TB cases by the year 2000 was reviewed comprehensively for the first time. It showed that 96 countries had implemented DOTS, and DOTS programmes demonstrated higher rates of cure than the previous standard of care (WHO 1998). A clearly articulated strategy and improved data collection in support of this strategy attracted additional organizations. For example, at this point, the U.S. Agency for International Development (USAID) became one of the central financial contributors to global TB efforts (Interview 5).
The number of organizations committed to addressing TB continued to grow, and as one respondent put it, WHO recognized that work could not go on ‘with WHO being the center of gravity of everything…We need[ed] many others to do their job [in order] to achieve something’ (Interview 5). To co-ordinate a larger response, greater collaboration between multiple organizations was seen as necessary. At the end of the 1990s, organizations such as the International Union Against TB and Lung Disease (the Union), the United States Center for Disease Control (CDC) and KNCV (a Dutch TB NGO) began to second people to the TB Program in WHO to facilitate co-ordination, and a series of meetings on future TB strategy were held.
In March of 1998, a meeting of the Ad Hoc Committee on the TB epidemic, organized by the TB programme, met in London to make recommendations on how to assist countries in achieving the targets. One of the primary recommendations resulting from this meeting suggested the urgent need for ‘a coordinated partnership’ of the WHO, the World Bank, development agencies, the Union, other NGOs and research institutions. Meetings held in Cambridge, Massachusetts in 1998 and Madrid, Spain in 1999, led to agreed changes to the DOTS model—re-formulated as DOTS-Plus—which included the addition of second-line TB drugs to the WHO Model List of Essential Drugs to address the issue of drug resistance. Much of the impetus for these meetings and for changing DOTS into DOTS-Plus came from outside WHO, from organizations such as Partners in Health and Médecins Sans Frontières, NGOs that criticized DOTS as ineffective in addressing MDR-TB as it was meant as a strategy for managing drug susceptible TB only. Then, in a meeting held in 2000 in Amsterdam, the fledgling WHO Stop TB Initiative brought together Ministers of Health from 20 of the 22 high TB burden countries, countries WHO had identified as having 85% of the global burden. This meeting produced the Amsterdam Declaration, a strong political statement that committed the signing governments to the implementation of the DOTS strategy along with the monitoring and evaluation of national TB programs in line with accepted WHO standards. All of these meetings helped to build goal consensus and to engage a broader range of organizations.
The mobilization of greater support for TB programs led to a new organizational structure for TB within WHO. In 2001, WHO created a Stop TB Department with two teams, one devoted to strategic and technical issues and one to focus on building a global partnership. In October 2001, the first Stop TB Partners’ Forum was held in Washington, D.C. Altogether, some 200 participants came from around the world, primarily government delegations and NGO representatives but also including foundations, intergovernmental organizations, public–private partnerships and bilateral donors. The Forum endorsed the Stop TB Partnership Framework, the Washington Commitment and the Global Plan to Stop TB (WHO 2001). It was at this meeting that the network officially coalesced. The Partnership Framework created the Partnership’s administrative structure, the Washington Commitment built on the Amsterdam Declaration to elicit political support for attaining the 2005 TB control targets, and the Global Plan outlined the strategies, priorities and resource needs of reaching these targets. The mandate of the Partnership was to lead global advocacy efforts and co-ordinate the activities of its partner organizations in order to reach these goals. Participating organizations signed onto these documents, and these three endorsements created the organizing structure for co-ordination and collaboration in TB, as well as consensus around targets and how they would be achieved.
The Global Plan, in particular, was acknowledged among most respondents as central to creating a cohesive community and co-ordinated work effort (Interviews 3–15). Partners in Health led the development of the first Global Plan in partnership with the WHO TB Department and in consultation with over 150 other individuals from various countries and organizations (PIH 2001). The Plan built not only on the DOTS strategy but also included sections devoted to HIV–TB co-infection, MDR-TB and research into developing new diagnostics, drugs and vaccines—addressing many of the areas DOTS had been criticized as neglecting.
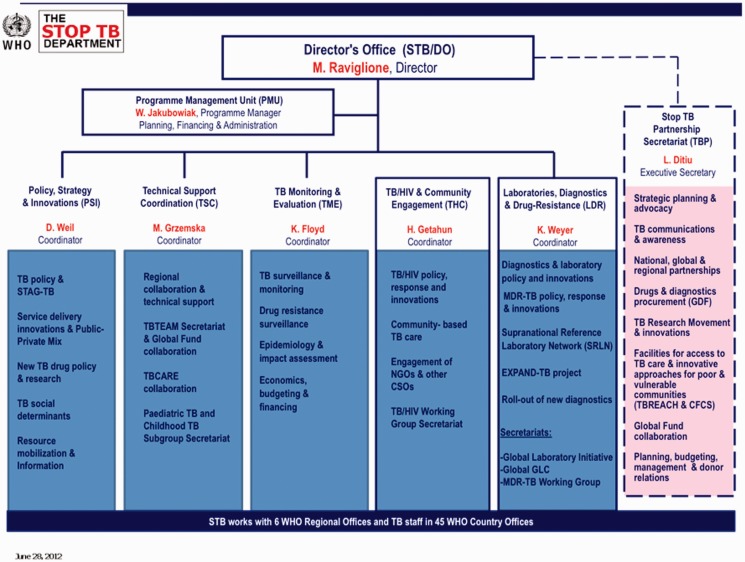
In terms of administration and governance, the Partnership Framework established the Stop TB Department as responsible for housing the Partnership Secretariat, with the WHO as host providing administrative and financial support. With offices on the same floor in the same building at WHO, the Executive Secretary of the Partnership reported to the Director of the TB Department. This administrative structure has continued until recently. Figure 1 shows the structure of the WHO TB Department as of 2014. The structure of the relationship between the department and Partnership Secretariat remained the same from 2001 until recently.
Figure 1.
Structure of the WHO TB Department WHO (2012c)
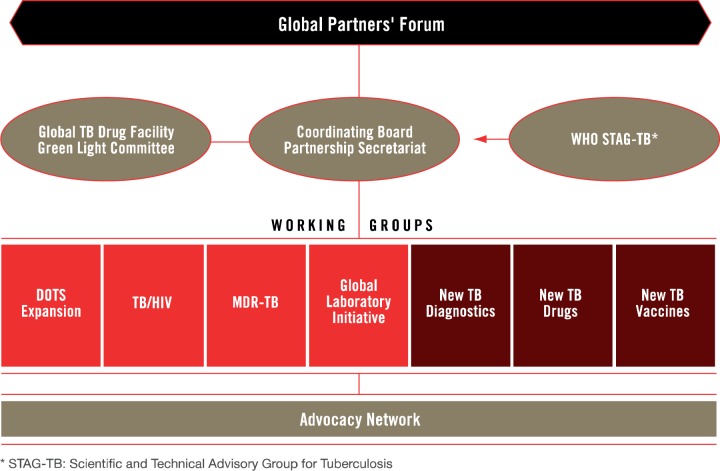
In addition to these administrative arrangements, participatory and advisory bodies such as the Co-ordinating Board, Working Groups and Partners’ Forum were created in 2001 to steer network activities, bring in new information and create venues for debate and discussion. These bodies have also stayed relatively constant, and Figure 2 provides an overview of these groups as they were organized up until 2014. The Co-ordinating Board was established to advise the Partnership, but had no legal authority (Caines et al. 2003). The Board’s mandate was to make decisions for the Partnership, provide leadership and direction, monitor the implementation of agreed policies and ensure co-ordination (Stop TB Partnership 2001; Caines et al. 2003). WHO has had a veto over Co-ordinating Board decisions to safeguard compliance with WHO rules and regulations, but has never used it (Interview 15). Both the Department and Partnership Secretariat, in practice, were accountable to the Board, but legally the Department was accountable to WHO (Stop TB Partnership 2001, Interview 5).
Figure 2.
Revised Structure of the Stop TB Partnership from the Global Plan 2011–2015 (2010)
The Working Groups were established to allow for more focused conversations on key research and implementation areas, and they created opportunities for greater participation and consensus building among network members. Participation in working groups was voluntary, and the work conducted on the initiative of working group members. Finally, the broadest representative body of the Partnership was the Partners’ Forum. The primary goal of the Forum was to consolidate and increase support for and commitment to the work of the Partnership. It was not set up to play a direct role in guiding activities but rather to rally members, meeting in 2001, 2004 and 2009.
While these participatory and advisory groups helped to guide activities, they are ultimately not responsible for making technical recommendations or carrying out suggested policies. Several technical bodies were also established. Within the TB Department is the Strategic and Technical Advisory Group for TB (STAG-TB) that provides ongoing technical and strategic advice. It is the STAG’s responsibility to make recommendations to WHO, and WHO typically takes these recommendations to update current policies and guidelines for best practice. Technical issues can come from working groups or other expert committees, but they go to the STAG for evaluation. The STAG consists of 18–22 members, many of whom may be members of working groups. Additionally, the STAG has a representative on the Co-ordinating Board. Next, to facilitate access to new technologies, the Partnership created the Global Drug Facility (GDF). Lack of access to TB drugs in countries motivated the creation of the GDF to serve as a mechanism for drug procurement and distribution. In 2014, the GDF delivered >24 million treatment courses to 133 countries (Stop TB Partnership 2014a).
In summary, until the Stop TB Partnership was established in 2001, the TB network was characterized by broad and loose relationships that ebbed and flowed over time. With the re-emergence of TB as a perceived crisis, TB experts at WHO initially dominated global activities. In the 1990s, other organizations were drawn into the global community through the scientific evidence demonstrating a link between the HIV epidemic and the resurgence of TB, the improved data on the cost-effectiveness of DOTS and the perceived momentum building globally to address TB through the efforts of organizations in the expanding central cluster. The composition of the network diversified, linking scientists, activists, donors and policymakers. The establishment of the Stop TB Partnership formalized the broader TB network, while the Partnership Framework’s administrative and governance structures institutionalized the leadership of the Co-ordinating Board, Partnership Secretariat and the WHO TB Department.
Network adaptation and effectiveness
Between 2001 and 2012, the TB network evolved from 200-plus organizations at the first Partners’ Forum into a network of ∼1600 organizations and individuals, demonstrating significant scalability (although many of these organizations were small, local NGOs, dormant at the global level). By 2012, the structure of the Partnership could be described as having a dense core group of organizations highly involved in network activities through the Co-ordinating Board and Working Groups, with a large peripheral network. Leadership of the network was highly centralized, with the Secretariat and the WHO TB Department as the primary institutions responsible for governing day-to-day activities. However, during this decade the network experienced various opportunities and challenges that created pressure for change (see Table 1 for a timeline of key developments).
Table 1.
Timeline of policy development, events, and network changes
| Date/environment | Policy developments/events | Network shifts and key actors |
|---|---|---|
| 1970s: period of complacency | TB neglected in West | The Union active in service delivery and some research |
| TB Control Programs in low/middle income countries | Small dept in WHO | |
| 1980s: TB gains attention | Link with HIV made | |
| 1989 | The Union’s Styblo demonstrates success in TB control through short course therapy | WHO’s TB Unit enlarged |
| 1990 | WHO recommends standardized short-course chemo therapy for developing countries | More staff appointed at WHO |
| 1991 | MDR-TB in New York hit headlines | |
| WHO and WB initiate China TB project testing implementation of short course therapy. | World Bank supports TB study in China | |
| World Health Assembly Resolution on TB | WHO | |
| 1993 | Mass media event in London in 1993 declaring ‘TB a Global Emergency’. | Advocacy expert hired by WHO TB Unit |
| World Bank Development Report says TB control a cost-effective measure | ||
| 1995 | DOTS marketed as strategy for addressing TB | WHO |
| Establishment of Global Surveillance and Monitoring System at WHO | WHO | |
| 1997 | First global monitoring report on TB published | |
| 1998 | Meeting of the Ad Hoc Committee in London | WHO initiative, with the Union, several donors, and high burden countries |
| Launch in Bangkok of the Stop TB Initiative | ||
| 2000 | Amsterdam Declaration to Stop TB. It called for action from ministerial delegations | Research institutions, donors, and NGOs become more involved in TB discourses |
| Millennium Development Goals established by UN, with TB as part of goal 6 | ||
| 2001 | The First Partners’ Forum of the Global Stop TB Partnership launched in Washington, DC | 200 partner organizations—any group can join existing network |
| The Global Plan to Stop TB 2001–2005 published as the overarching framework of the Stop TB Partnership’s combined actions. | ||
| 2002 | Launch of Global Fund to Fight AIDS, Tuberculosis and Malaria. | Global Fund brings significant resources to TB |
| WHO publishes an expanded DOTS framework addressing the issues of TB/HIV and drug resistance | WHO | |
| 2003–2004 | New Director of the TB Department, Dr. Mario Raviglione, and new Executive Secretary of the Partnership, Marcos Espinal | WHO |
| 2006 | New Global Plan 2006–2015 | Many different groups consulted over Plan |
| New Stop TB Strategy enhancing DOTS | ||
| 2009 | Beijing Ministerial Conference on MDR- and XDR-TB | WHO, Gates Foundations, MOHs from high-burden countries |
| 2010 | WHO endorses a new and novel rapid test for tuberculosis (TB) called the Gene Expert Diagnostic test. | Over 1600 partner organizations |
| 2010–2011 | Selection of new Executive Secretary of Stop TB Partnership | WHO and the Partnership Co-ordinating Board Selection Committee |
| 2013 | Co-ordinating Board meeting that includes a discussion of alternative hosting arrangements | Partnership Co-ordinating Board |
| 2014 | Decision to move Partnership Secretariat to UNOPS | Partnership Co-ordinating Board |
For example, as a consequence of the United Nation’s MDGs and several initiatives to raise significant funds to help attain these goals, there was a huge rise in funding for health from the early 2000s (Walt et al. 2012). Annual development assistance for health increased from $2.5 billion in 1990 to ∼$16 billion in 2006—doubling between 2000 and 2009 (Taskforce on Innovative International Financing for Health Systems 2009), and much of this funding went to support programs on AIDS, TB and malaria. In 2002, the Global Fund to Fight AIDS, TB and Malaria was launched, and became the largest funder of TB work globally, making up 82% of external funds for TB control (WHO 2011). While TB is technically part of MDG 6 (to combat HIV/AIDS, malaria and other diseases), it is not mentioned specifically until indicators 6.9 and 6.10, and initially, the Global Fund directed most of its funds to the AIDS response, with only 19% for TB (The Global Fund 2003). However, it is likely that the advocacy and data surrounding DOTS, the World Bank’s assessment of DOTS’ cost-effectiveness, TB’s scientifically established link with AIDS, and WHO’s active TB program helped get TB on the agenda of global health policymakers (Murray and Lopez 1996).
The process leading up to the 2000 MDGs has been criticized for its lack of consultation, with a small group of bureaucrats finalizing the list of goals based on objectives that had reasonably robust and comparable data, and on issues that would easily be accepted by nearly 200 governments (Fukuda-Parr and Hulme 2011). Members of the TB Department at WHO were not involved in the inclusion of TB in the health goals (Interview 5). As for the Global Fund priorities, WHO Director-General Gro Harlem Brundtland, her Chief of Staff David Nabarro, and several other high-level staff advocated for the inclusion of TB and malaria in a fund to address diseases of poverty, participating in many of the planning meetings, including as host of one of these meetings in June 2001 (Lidén 2013). At this point, the TB network was still being formalized.
The inclusion of TB in the MDGs and Global Fund priorities was a significant opportunity for advancing attention, resources and policy implementation. However, the policy environment was not without complications. An evaluation of the Stop TB Partnership in 2003 commented that the creation of the Global Fund intensified competition for financial resources and increased uncertainty over funding flows for the Partnership (Caines et al. 2003). Recognizing that the Global Fund offered great potential for furthering the goals and objectives of the Partnership, an increasing number of meetings took place between the Partnership Secretariat and Co-ordinating Board with the Secretariat of the Global Fund. Initially, the Global Fund was asked to send an observer to Co-ordinating Board meetings, and in 2004, this position became permanent (McKinsey & Co. 2008). Also in 2003, a formal Memorandum of Understanding was developed between the Partnership and Global Fund, delineating who was responsible for what, and how they would co-ordinate efforts. For example, it was agreed that the Partnership would provide second-line drugs to Global Fund grantees through its Green Light Committee procurement agent (Caines et al. 2003).
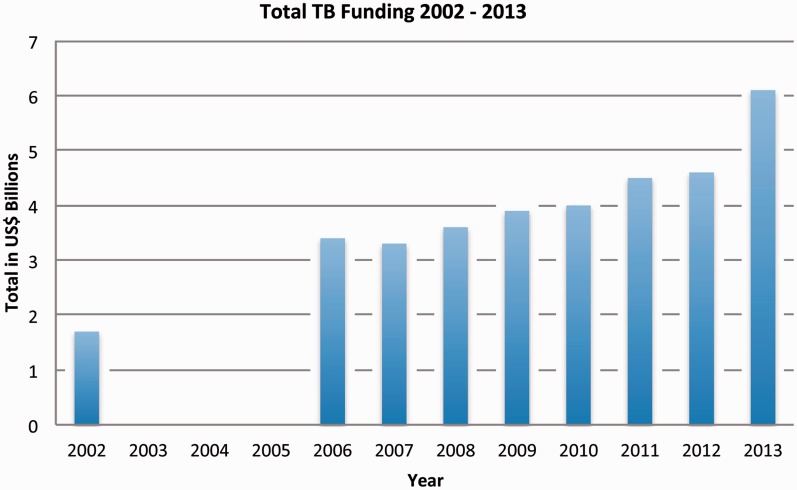
Over time, this relationship continued to evolve from one of competition to one of collaboration, with representatives from the Global Fund taking on important leadership roles in the Partnership, such as the Chair of the Co-ordinating Board in 2011. This is a prime example of how the network adapted to include new organizations in its existing systems. The inclusion of the Global Fund was critical as the next decade saw a dramatic rise in funding for TB, largely due to its activities. In 2002, external funding for TB was ∼US$1.3 billion in the 22 high-burden countries, and by 2012, it had expanded to US$3.3 billion (WHO 2011). As shown in Figure 3, total funding for TB globally increased from US$1.7 billion in 2002 to US$6.1 billion in 2013. As much of the external funding is directed through the Global Fund, it is difficult to tease out the effect of the network from the efforts of the Global Fund itself (which might be acting as a member of the network or as an organization pursuing its own distinct goals), but it is clear that engagement with the Global Fund has been of great import. It is also important to point out that the majority of funding for TB control (86%) has continuously come from domestic sources, with the largest share of funding going towards TB diagnosis and treatment with first-line drugs—part of the DOTS strategy (WHO 2012a). These data suggest that the network’s promotion of DOTS successfully contributed to the adoption of the DOTS strategy at the national level, increasing funding for DOTS over time. However, a funding gap of US$2 billion remains (WHO 2014).
Figure 3.
Total Global Funding for TB from 2002 to 2013
(Total TB Funding data are from the annual WHO Global TB Control Reports, data missing for 2003–2005)
Even while adapting to include new members in network governance, key leadership positions stayed largely the same. In 2003 and 2004, a new Director of the TB Department, Mario Raviglione, and a new Executive Secretary of the Partnership, Marcos Espinal, took on these steering roles. Dr. Espinal served as the Executive Secretary until 2010, and at the time of research (2014), Dr. Raviglione continued in his role as Director. Both Dr. Raviglione and Dr. Espinal came from other positions at WHO where they had been working since 1991 and 1997, respectively. Both leaders also saw DOTS as the backbone of TB control. The close working relationship between the two, and the length of their tenures, was credited with creating stability and consensus within the Partnership’s governance structure (Interview 5, 8, 15). However, the closeness of the Partnership to the Department was also seen as hampering Partnership activities (Interview 7, 12, 13), delaying ideas from partner organizations, and placing too much attention on Ministries of Health at the country level.
As network activities progressed, there were internal calls for modifications to its goals for treatment scale-up and mortality reduction. In 2004, the Co-ordinating Board tasked the WHO TB Department with updating the Global Plan. The Department led the revision, consulting global and regional stakeholders (Interview 8). There were tense discussions over whether to make the plan a technical or advocacy document. The final compromise was an advocacy piece, but one that contained detailed epidemiological data (Interview 8) and clear, achievable targets (Interview 5). Seen largely as a success, the Global Plan to Stop TB 2006–2015 was launched at the World Economic Forum in Davos in 2006, where Bill Gates committed $600 million to the Stop TB efforts (Weber 2006).
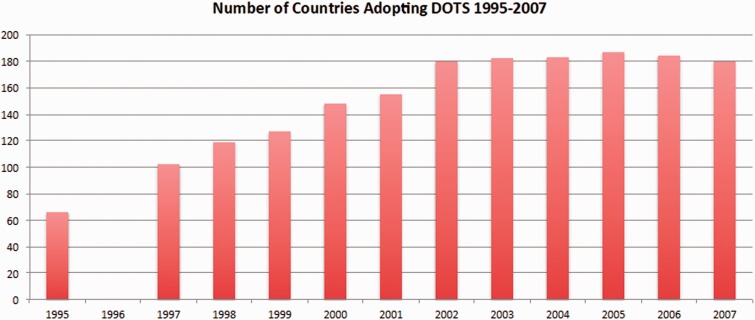
The debate over what changes to make to the Global Plan also appeared in discussions around the DOTS strategy. At a Partnership symposium held before the 2006 annual TB conference, Mark Harrington, the Executive Director for the Treatment Action Group told the participants ‘… you are more invested in the DOTS model—which has failed [to contain] multidrug-resistant TB…than you are in saving lives’ (Smart 2006). Summarizing the opinion of activists, Smart (2006) observed ‘that the model virtually ignores most people with HIV-related TB, who often have either extrapulmonary or smear-negative TB, because those conditions are generally non-infectious (though no less fatal), and people with MDR-TB because they were too difficult to treat…essentially leaving them to die’. Among some of the global activist organizations in the Partnership, dissension was growing over the perceived neglect of MDR and HIV-related TB. Even though the DOTS strategy had expanded both in 1998 and 2002 to bring greater attention to TB/HIV and MDR-TB, activist groups did not see these changes as leading to more aggressive action. These critiques led to another reassessment of DOTS, which at this time had been adopted in 180 countries (WHO 2009) (Figure 4).
Figure 4.
Number of Countries Adopting the DOTS Policy from 1995–2007
(Policy adoption data come from the annual WHO Global TB Control Reports, data missing for 1996)
A new ‘Stop TB Strategy’ was launched in 2006 as a response to these challenges, endorsed by around 400 people at a Stop TB Partnership meeting (Raviglione and Uplekar 2006). Most of the participants were TB program managers, technical and financial partners, researchers, HIV/AIDS experts, health activists and WHO staff. The strategy included DOTS expansion and enhancement, but emphasized addressing TB/HIV and MDR-TB, contributing to health system strengthening, engaging with health care providers across various sectors, empowering people with TB and enabling and promoting research. Maintaining DOTS as the backbone of TB control was supported by those at WHO, but continued to frustrate a sub-cluster of organizations such as Médecins Sans Frontières, Partners in Health and the Treatment Action Group. In 2010, the Global Plan was updated again to address the continuing concerns of some of the partner organizations. The new plan set more ambitious targets and, for the first time, identified all the research gaps to be filled (Stop TB Partnership 2010). While these discussions contributed to adaptation in strategy, they also highlighted a consistent under-current of disagreement over the primary goals and solutions of the network, and concern that even though adjustments had been made, priorities had not shifted as much as the dissenters would have liked.
One issue underlying these internal network debates was the increasing number of reported MDR and XDR-TB cases that reached almost 480 000 worldwide in 2013 (WHO 2014). Part of the conflict centred on the lack of data. Data collection on MDR and XDR-TB has lagged behind other data gathering. While assessment of global trends in MDR began between 1996 and 1999 (Espinal et al. 2001), and while countries were asked to report data on MDR and XDR-TB since 2003 (WHO 2004), few have consistently complied. It was not until 2010 that the network had a better idea of the growth of these epidemics (Nathanson et al. 2010). In addition to data problems, the cause of increasing MDR-TB cases was debated, with some pointing to the poor management of DOTS programs (Smart 2006; Anand and McKay 2012), but others suggesting that three quarters of the estimated cases of MDR-TB occurred in previously untreated patients, implying that the spread of infection was not through DOTS programs (Nathanson et al. 2010).
The contestation surrounding DOTS has continued, with considerable differences of opinion over its effectiveness. One analysis demonstrated that between 1995 and 2012, 56 million people were successfully treated for TB in countries that had adopted the DOTS/Stop TB strategy, saving an estimated 22 million lives (WHO 2013). Overall, the global mortality rate fell by 45% from 1990 to 2013, and the number of incident cases has been falling slowly (at an average rate of 1.5% per year) since 2000 (WHO 2014). However, there was also uncertainty about the impact of DOTS on the burden of TB. Another study of predictors of TB case notification trends between 1997 and 2006 suggested that changes in TB incidence were more strongly associated with biological, social and economic factors than with National TB Program performance (Dye et al. 2009; Lönnroth et al. 2009). Additionally, different groups within the network made different claims for success or failure of TB efforts. In 2011, a group from Harvard, plus several NGOs, commented that ‘In the past decade the number of new cases of TB worldwide has barely declined, and the number of deaths remains catastrophic…’ (Keshavjee et al. 2011). There is a divide between those who focus on the improvement of DOTS over the previous standard of care, and those who draw attention to slow progress in the elimination of deaths from TB.
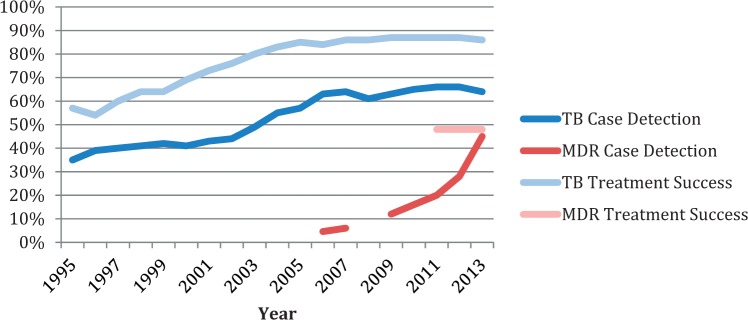
Regarding adaptations made to the DOTS strategy and Global Plan, data indicate that progress is being made on MDR-TB, HIV/TB and research into new drugs, vaccines and diagnostics; however, the delay in attention is apparent and progress continues to lag. For instance, as shown in Figure 5, global case detection for drug-susceptible TB has increased from 35% in 1995 to 64% in 2013. Meanwhile, data on MDR case detection was not measured and reported globally until 2006, and in that time, it has increased from 4.6 to 45% (WHO 1998–2014). MDR case detection has increased significantly but continues to fall far short of global targets for detecting 70% of all TB cases. As for treatment success rates, global treatment success for drug-susceptible TB has increased from 57% in 1995 to 86% in 2013, whereas MDR treatment success was not reported until 2011 and has stayed consistently at 48% for the past 3 years (WHO 2014). Treatment for MDR-TB is expensive and complex, with long treatment regimens (>20 months) that are poorly tolerated and substantially less effective than first-line treatment (Abubakar et al. 2013).
Figure 5.
Drug Susceptible and Drug-Resistant TB Case Detection and Treatment Success 1995–2013
(Case detection and treatment success data come from the annual WHO Global TB Control Reports)
Until the approval and roll-out of GeneXpert in 2010, a new diagnostic technology for detecting drug resistance, and two new drugs approved since 2012 for treating MDR-TB (WHO 2014), it had been 40 years since a new tool was developed for managing TB (Lessom 2014, Interview 4). Access to the new drugs remains limited, and while efforts to develop new TB diagnostics, drugs and vaccines have intensified, with 10 new or repurposed drugs currently in late phases of clinical development and 15 vaccine candidates in clinical trials, the decades of neglect have fueled criticisms of the lack of innovation and slow progress (Interviews 4, 7, 12, 13). Indeed, investments in TB research continue to fall far short of goals outlined in the Global Plan. The Treatment Action Group started to track funding for TB research and development in 2005, finding that it has increased from ∼US$357 million in 2005 to US$627 million in 2012 (Frick and Jiménez-Levi 2013). However, funding declined by US$30 million from 2011 to 2012, and the gap between funding needs and investments grew to ∼US$1.4 billion.
Internal debate has continued over the post-MDG goals for TB. The network was unified in its desire to get TB included with greater prioritization in the post-2015 development agenda, but what this goal will be was disputed. One possible target, initially favoured by the WHO TB Department, was to cut the projected 2015 annual deaths in half by 2025. Dr. Raviglione called a 50% target feasible yet challenging enough to drive change (Maxmen 2013). However, activist NGOs, such as the Treatment Action Group, and the current Executive Secretary Dr. Lucica Ditiu backed a push for more ambitious targets such as zero new infections and zero deaths from TB, and are unhappy with the perceived ‘lack of ambition that has marred the fight against tuberculosis all along’ (Maxmen 2013). To develop consensus, the WHO and Stop TB Partnership called an additional consultation in Geneva in February 2013. The aspirational goal of ‘zero TB deaths, zero TB disease and zero suffering’ by 2050 was agreed to, along with a set of interim targets for the year 2025 (Raviglione and Ditiu 2013).
Managing differences of opinion and reaching consensus has been a primary challenge to network governance. These differences became more manifest with a change in leadership at the Partnership in 2011, creating greater momentum for altering the governance structure of the network. A new Executive Secretary, Dr. Ditiu, was selected, and this led to a change in the working relationship between the TB Department, the Secretariat, and WHO. Dr. Ditiu’s leadership was applauded by those who saw her as ‘innovative’ (Interview 13) and a ‘breath of fresh air’ (Interview 1), but was also questioned by some who saw her as upsetting the balance and stability that had existed since the Partnership was established (Interview 8, 14, 15). One of her more controversial decisions was to push for a more distinct separation between the Department and the Partnership, in response to some partner complaints that the Partnership prioritized WHO interests over others (Interview 4). For example, there was a series of comments and editorials published in ‘The Lancet’, some critical of the Partnership’s inadequacies in addressing MDR-TB, its slow progress in facilitating new technologies, and disparaging of perceived conflicts of interest arising from its closeness to WHO, and others defending the relationship between the Partnership and TB Department and the efficiency of Partnership’s efforts (Ditiu 2011; Keshavjee 2011; Espinal 2012; Raviglione et al. 2012).
In late 2012, at the request of the Co-ordinating Board, an external review team was established to assess the hosting situation at WHO as well as three alternative hosting arrangements (at the United Nations Development Programme [UNDP], the United Nations Office for Project Services [UNOPS], and The Union; Boutel et al. 2013). Presented at the July 2013 Co-ordinating Board meeting, the review report emphasized differences in financial costs and transparency across the four organizations, as well as the amount of autonomy provided to the Secretariat. In July 2014, the Board decided to move the Partnership Secretariat to UNOPS early in 2015, stating that the Partnership will be better able to fulfill its mandate of leading global advocacy efforts, raising funding and action against TB and co-ordinating the efforts of all its partner organizations (Stop TB Partnership 2014c). This potential change is controversial, however, the decision was ultimately supported and facilitated by WHO. The organization will remain a central partner, a member of the Co-ordinating Board, and the primary provider of data and policy norms and standards. The outcome of this change in administrative and governance structures on global TB efforts is still playing out.
In summary, while network strategies have adapted over time, network administration and governance have remained largely unchanged until recently. The concern among a cluster of network members that the system of network administration and governance established in 2001 constrained the ability of the Partnership to represent the voices of all network members and to act quickly and with flexibility in response to emerging issues contributed to the pressure for changing the hosting of the Partnership. Adaptations to strategy have led to improvements in policies and outcomes for HIV/TB, MDR-TB and research into new technologies, but these changes happened later and slower than what some network members advocated, and they are still not at the level needed. Meanwhile, what adaptations in network governance mean for the network’s influence on policies and health outcomes is still to be seen.
Discussion
Within the network, the WHO TB Department has remained a key figure, even with the creation of the Partnership and its various participatory and governance bodies. The centrality of the TB Department and the continuity of its leadership shaped both the governance of the network and the activities on which it focused. The mode of governance can be described as a hybrid between a lead organization and a network administrative organization model (Provan and Kenis 2008) with a distinct network secretariat established but hosted within the WHO. Additionally, the DOTS strategy has remained the foundation of network promoted TB control policies, even as the strategy has changed over time in response to new data and differences of opinion within the network. The initial choices of the network have remained largely constant until recently, with several incremental shifts in the area of strategy, and one dramatic shift in governance with the Secretariat moving to UNOPS in 2015. The internal pressure for this change was largely the result of differences of opinion that have existed since the network was formed, a changing epidemic and a system that some see as unresponsive to new ideas and calls for change.
While the internal debates have been present since early on, the TB network has successfully grown, demonstrating significant scalability over time. Through its activities and a supportive policy environment, the network quickly expanded. Among the core group of organizations, the centrality of WHO, though contested, may have helped to extend and activate the network and to accelerate the diffusion of the DOTS strategy. For example, the resources WHO brought to the network were quite substantial, particularly in terms of technical expertise and normative power. Interviewees all agreed that WHO was seen as the global leader in establishing norms and policy. WHO’s mandate was also to represent the concerns of member states, giving further legitimacy to its authority. By recognizing the need to create a larger network, WHO helped to bring in additional resources, to share and disseminate research, to avoid duplication in effort, and to accelerate momentum. The expanded network further legitimized WHO’s intervention strategy because it increased the number of independent actors committed to advocating and implementing DOTS.
DOTS policies have now spread to over 180 countries and funding for TB has increased over time, with much of it going to DOTS based activities. The leadership of WHO in creating a broader initiative around DOTS led to an organizational entity (the Partnership) and message that appealed to donors and national-level organizations, actors who may not have responded as strongly to a WHO initiative. The leadership of the WHO TB Department contributed to the perceived legitimacy of the Partnership, and the Partnership increased the perceived legitimacy of the Department. In this manner the relationship between the two was mutually beneficial. The stability of this leadership and the public positioning of the DOTS strategy over time may have helped contribute to the network’s scalability.
That the TB Department’s role had not changed over the past decade was largely due to the consistency in leadership, the value many members saw in having WHO as an influential partner, and the costs involved in changing a structure that had been operating for a decade. In the debate about whether or not the Partnership should separate more clearly from the TB Department, the costs of the transition were compared with the potential gains. How far donors would support a change in control over resources that a separation entails is not clear. The TB Department was highly respected among donors and academics for its scientific approach to analysis and review of TB data, its expert knowledge and the leadership of its Director, Dr. Raviglione (Interviews 3, 10, 11, 14).
Consistent leadership may have helped the network expand, but this stability also challenged the network’s adaptability, both with regard to political demands from some partners (the core activist or NGO groups) and to the changing epidemic. An important characteristic of TB is that MDR- and XDR-TB can form, and there are a growing number of reported cases that are extremely challenging to treat with current practices and drugs. These changes in the disease require new diagnostics, new drugs, better integration of diagnostics and treatment and better monitoring of patient care—to name a few. At issue is whether or not the TB network has kept up with these required changes because of the institutionalization of DOTS and the centrality of the TB Department. Perspectives on this issue vary dramatically within the network, and the policy and public health outcomes data can be used to support either perspective. Improvements are being made, but implementing policies on drug-resistance and HIV/TB integration, and investing in new research and development for TB has lagged, and there is still much to accomplish.
While highly centralized networks may be inclusive of new members, they are not known for integrating new ideas and preferences (Provan and Kenis 2008; Burt 1992). This is not universally the case in the TB network. The participatory bodies of the Partnership contributed to changing external strategies, such as the shift from DOTS to the Stop TB Strategy and the periodic updates to the Global Plan. New research does make its way into the decision-making and consensus-building processes at the core. However, a sub-cluster of NGOs in the core group—particularly those concerned with HIV and the growth in MDR-TB—perceive that the Partnership largely functioned in a top-down manner, with WHO bureaucracy hampering the speed of progress. One of the main examples critics point to is the continued reliance on existing drugs to control a disease that in drug-resistant forms is no longer controllable (Interviews 4, 12, 13). For the most part, the core group was a closely knit network of technical experts, donors and some health ministries, with a sub-group of international NGOs attempting to push the Partnership in new directions. The upcoming move to UNOPS may change these dynamics.
The evolution of the TB network demonstrates that scalability and adaptability are processes in conflict with one another. Stability in organizational structure and the ability to expand quickly did not lend itself to adaptation in structure, but instead it contributed to path dependency. Network governance remained largely the same, strategies were adapted to include other priorities, but DOTS continued as the foundation of global TB control. Once the network established a particular organizing structure and primary strategy, entrenchments of these institutional arrangements frustrated an easy reversal of the initial choices even with internal and external pressures for change.
Overall the TB network was successful in recruiting new members, sustaining attention and resources for TB, and in scaling-up the primary interventions strategy—DOTS. In this sense, the network experienced a surge of success over the past decade-and-a-half. However, it cannot be assumed that this success will be maintained. The disease is changing, the pathogens mutating, and reported cases of MDR- and XDR-TB are increasing. These present huge challenges at both the global and local, country program levels. Data on MDR- and XDR-TB are improving, research into new technologies are advancing, but many organizations in the core group are frustrated at the perceived slow pace of progress. It is unclear how changes in the governance of the network and its goals and activities will influence the outcomes of the network, and its future impact on TB mortality and morbidity.
Implications
The primary finding from this case study is that there are important attributes of networks that may differentially influence network effectiveness over time. The stability necessary for scalability to take place may be an important factor for networks in the early stages of network development, with centralized authority contributing to durability. Having a centralized core group and a strategic brand helped the network to coalesce around the DOTS strategy. While networks are often thought of as loose and decentralized, they can be hierarchical, and a strong core that provides leadership and common purpose may help to make them effective. This may be especially the case when trying to raise the political priority of an issue. At an early stage, greater centralization in structure and strategy may make networks more effective.
But as the network ages, the ability for it to adapt increases in importance. Adaptation becomes key as membership expands and diversifies, and other organizations in the network become aware of, and challenge, the power relationships institutionalized in governance structures. Increasing diversity can lead to tensions over who controls decision-making processes, and these internal debates can lead to the questioning of the network’s organization. How a network reaches decisions and how it builds consensus are two mechanisms that facilitate adaptability. These mechanisms may also need to change over time for a network to continue to function well as circumstances alter. Differences over how to approach the problem of TB have been both a challenge and an opportunity for the network. While disagreement can be destabilizing if managed poorly, it can also serve to bring in new ideas and to create internal pressure to improve. If managed well, contestation can contribute to adaptability and sustainability—helping to create a more appropriate response to a disease that continues to evolve. If managed poorly, disagreement could lead to the disintegration of the network. This has yet to be resolved in relation to the current TB network.
Finally, for networks with lead or network administrative organizations, adaptation may be more difficult if the centralized leadership does not want to change. This finding raises an important question regarding institutionalization in networks. While institutionalized rules or norms may resist change, are some rules and norms more resistant than others? For example, as the TB network demonstrates, changes to strategies are easier than changes to governance. The financial cost of change is likely an explanation for this difference, but the cost of altering power relations must also be considered. It is possible that networks that are initially more highly centralized may be more resistant to pressures for decentralization than vice versa. The TB case provides support for the former, but this issue should be looked at across a greater number of networks with different governance systems and structural arrangements.
Regarding the TB network itself, addressing a complex and changing disease such as TB demands a sensitive and responsive approach that is able to adapt to different settings, to shifting epidemiologies and to the mutational capacity of the disease. Success is always temporary, and global efforts have to be met with local, country efforts. The implication for the network is that it must include both global and local members, each playing their own parts. The upcoming changes in network structure away from the current centralized core at WHO towards a more independent Partnership hosted at UNOPS should consider the long-standing challenges to the network and the new complications this move may create, including: how the move will affect ongoing relationships within the network; how it will affect resources for the network and for TB; and how it will change decision-making and consensus-building processes with an eye towards managing enduring disagreements. Reducing the administrative costs of the Partnership, improving financial transparency and creating a more autonomous Secretariat may help to strengthen the effectiveness and sustainability of the TB network into the future, but without a clear strategy for engagement and the management of diverse opinions, governance struggles may continue—with implications for long-term network effectiveness.
Supplementary Material
Acknowledgements
The authors thank John Porter at the London School of Hygiene and Tropical Medicine for his insights and assistance and members of the GHAPP team for their valuable feedback on drafts of this article. The authors would also like to thank our interviewees for their willingness to participate in our study and to provide us additional feedback. Without their openness and comments, our research project would not have been feasible.
Supplementary data
Supplementary data are available at HEAPOL online.
Funding
The Global Health Advocacy and Policy Project (GHAPP) is funded by the Bill and Melinda Gates Foundation [OPPGH4831].
Conflict of interest statement. None declared.
Ethical approval
The authors cleared the study protocol through the Institutional Review Boards of American University and Syracuse University, which granted the study exempt status, as it focused on public policy and was deemed to pose minimal risk to informants.
References
- Abubakar I, Zignol M, Falzon D, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infectious Diseases. 2013;13:529–39. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- Anand G, McKay B. How fight to tame TB made it stronger. The Wall Street Journal. 2012 http://www.wsj.com/articles/SB10001424127887324894104578115232206834770, accessed 1 May 2015. [Google Scholar]
- Anonymous. The global challenge of tuberculosis. The Lancet. 1994;344:277–9. [PubMed] [Google Scholar]
- Bennett A. Process tracing and causal inference. In: Brady H, Collier D, editors. Rethinking Social Inquiry: Diverse Tools, Shared Standards. 2nd. Lanham, MD: Rowman & Littlefield Publishers; 2010. [Google Scholar]
- Boutel T, Vijay A, Szabo R. Independet Review of Hosting Arrangements: Stop TB Partnership. Geneva, Switzerland: Stop TB Partnership; 2013. [Google Scholar]
- Burt RS. Structural holes: The social structure of competition. Cambridge, MA: Harvard University Press; 1992. [Google Scholar]
- Buse K, Harmer AM. Seven habits of highly effective global public-private health partnerships: Practice and potential. Social Science & Medicine. 2007;64:259–71. doi: 10.1016/j.socscimed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Buse K, Tanaka S. Global Public-Private Health Partnerships: lessons learned from ten years of experience and evaluation. International Dental Journal. 2011;61:2–10. doi: 10.1111/j.1875-595X.2011.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse K, Walt G. Global public-private partnerships: part I-a new development in health? Bulletin of the World Health Organization. 2000;78:549–61. [PMC free article] [PubMed] [Google Scholar]
- Caines K, Biritwum R, Cameron N. Independent External Evaluation of the Global Stop TB Partnership. London: IHSD; 2003. [Google Scholar]
- Ditiu L. A new era for global tuberculosis control. The Lancet. 2011;378:1293. doi: 10.1016/S0140-6736(11)61567-5. [DOI] [PubMed] [Google Scholar]
- Dye C, Lönnroth K, Jaramillo E, et al. Trends in tuberculosis incidence and their determinants in 134 countries. Bulletin of the World Health Organization. 2009;87:683–91. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal MA, Laszlo A, Simonsen L, et al. Global trends in resistance to antituberculosis drugs. New England Journal of Medicine. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- Espinal MA. WHO’s relationship with the Stop TB Partnership. The Lancet. 2012;379:612. doi: 10.1016/S0140-6736(12)60158-5. [DOI] [PubMed] [Google Scholar]
- Finnemore M, Sikkink K. International norm dynamics and political change. International Organization. 1998;52:887–917. [Google Scholar]
- Frick M, Jiménez-Levi E. Report on Tuberculosis Research Funding Trends 2005–2012. New York, NY: Treatment Action Group; 2013. [Google Scholar]
- Fukuda-Parr S, Hulme D. International norm dynamics and the “end of poverty”: understanding the Millennium Development Goals. Global Governance. 2011;17:17–36. [Google Scholar]
- Hong L, Page SE. Groups of diverse problem solvers can outperform groups of high-ability problem solvers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16385–9. doi: 10.1073/pnas.0403723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUATLD. 2010. History of the Union, Paris, France: International Union Against Tuberculosis and Lung Disease. Available at: http://www.theunion.org/index.php/en/who-are-we/history-of-the-union, accessed 15 February 2012.
- Kahler M. Networked Politics: Agency, Power, and Governance. Ithaca, NY: Cornell University Press; 2009. [Google Scholar]
- Katzenstein PJ. The Culture of National Security: Norms and Identity in World Politics. New York, NY: Columbia University Press; 1996. [Google Scholar]
- Keck ME, Sikkink K. Activists Beyond Borders: Advocacy Networks in International Politics. Ithaca, NY: Cornell University Press; 1998. [Google Scholar]
- Keshavjee S, Harrington M, Gonsalves G, et al. Time for zero deaths from tuberculosis. The Lancet. 2011;378:1449–50. doi: 10.1016/S0140-6736(11)61521-3. [DOI] [PubMed] [Google Scholar]
- Kingdon J. Agendas, Alternatives and Public Policies. Boston and Toronto: Little, Brown and Company; 1984. [Google Scholar]
- Lawn SD, Zumla AI. Tuberculosis. The Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- Lee K, Buse K. Health Policy in a Globalising World. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Lessem E. 2014. Tuberculosis drug development hobbles forward. In: Clayden et. al. HIV i-Base & Treatment Action Group (eds). Pipeline Report, pp. 197–216. [Google Scholar]
- Lidén J. The Grand Decade for Global Health: 1998–2008. Centre on Global Health Security Working Group Papers. London, UK: Chatham House; 2013. [Google Scholar]
- Lönnroth K, Jaramillo E, Williams BG, et al. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Social Science & Medicine. 2009;68:2240–46. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Marsh D, Smith M. Understanding policy networks: towards a dialectical approach. Political Studies. 2000;48:4–21. [Google Scholar]
- Maxmen A. Ahead of WHO meeting, experts clash over tuberculosis targets. Nature. 2013;19 doi: 10.1038/nm0213-115. http://www.nature.com/nm/journal/v19/n2/full/nm0213-115.html, accessed 1 May 2015. [DOI] [PubMed] [Google Scholar]
- McAdam D, Tarrow S, Tilly C. Dynamics of contention. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- McInnes C, Kamradt-Scott A, Lee K, et al. Framing global health: The governance challenge. Global Public Health. 2012;7:S83–94. doi: 10.1080/17441692.2012.733949. [DOI] [PubMed] [Google Scholar]
- McKinsey & Co. K. Independent Evaluation of the Stop TB Partnership. London, UK: McKinsey and Company; 2008. [Google Scholar]
- Murray JL, Lopez AD. Evidence-based health policy—lessons from the global burden of disease study. Science. 1996;274:740–3. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Nair N, Wares F, Sahu S, et al. Tuberculosis in the WHO South-East Asia Region. Bulletin of the World Health Organization. 2010;88:164. doi: 10.2471/BLT.09.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson E, Nunn P, Uplekar M, et al. MDR tuberculosis—critical steps for prevention and control. New England Journal of Medicine. 2010;363:1050–1058. doi: 10.1056/NEJMra0908076. [DOI] [PubMed] [Google Scholar]
- Ogden J, Walt G, Lush L. The politics of “branding” in policy transfer: the case of DOTS for tuberculosis control. Social Science & Medicine. 2003;57:179–188. doi: 10.1016/s0277-9536(02)00373-8. [DOI] [PubMed] [Google Scholar]
- Ostrom E. Institutional rational choice: an assessment of the institutional analysis and development framework. In: Sabatier PA, editor. Theories of the Policy Process. Boulder, CO: Westview Press; 2007. pp. 21–64. [Google Scholar]
- Oxlade O, Murray M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS ONE. 2012;7:e47533. doi: 10.1371/journal.pone.0047533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablos-Méndez A, Raviglione M, Laszlo A, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. New England Journal of Medicine. 1998;338:1641–9. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- Page SE. The Difference: How the Power of Diversity Creates Better Groups, Firms, Schools, and Societies (New Edition) Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- Pierson P. Increasing returns, path dependence, and the study of politics. American Political Science Review. 2000;94:251–67. [Google Scholar]
- PIH. The Global Plan to Stop TB, 2001–2005. Partners in Health; 2001. [Google Scholar]
- Porter JDH, Grange JM. Tuberculosis: An Interdisciplinary Perspective. River Edge, NJ: Imperial College Press; 1999. [Google Scholar]
- Powell W. Neither Market nor Hierarchy: Networks Forms of Organization. Research in Organizational Behavior. 1990;12:295–336. [Google Scholar]
- Provan KG, Kenis P. Modes of network governance: structure, management, and effectiveness. Journal of Public Administration Research and Theory. 2008;18:229–52. [Google Scholar]
- Ramon-Pardo P, Jacquet V, Armengol R, et al. Scaling up collaborative TB-HIV activities in Latin America. International Journal of Tuberculosis and Lung Disease. 2008;12:S51–53. [PubMed] [Google Scholar]
- Raviglione M, Nunn P, Floyd K, et al. WHO’s relationship with the Stop TB Partnership. The Lancet. 2012;379:611–2. doi: 10.1016/S0140-6736(12)60157-3. [DOI] [PubMed] [Google Scholar]
- Raviglione MC. The TB epidemic from 1992 to 2002. Tuberculosis. 2003;83:4–14. doi: 10.1016/s1472-9792(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Raviglione M, Ditiu L. Setting new targets in the fight against tuberculosis. Nature Medicine. 2013;19:263. doi: 10.1038/nm.3129. [DOI] [PubMed] [Google Scholar]
- Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948–2001. The Lancet. 2002;359:775–80. doi: 10.1016/s0140-6736(02)07880-7. [DOI] [PubMed] [Google Scholar]
- Raviglione MC, Uplekar MW. WHO’s new Stop TB Strategy. Lancet. 2006;367:952–5. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- Reingold A, Gordon A. Infectious diseases. In: Merson M, Black R, Mills A, editors. Global Health: Diseases, Programs, Systems and Policies. Burlington, MA: Jones & Bartlett Learning; 2012. [Google Scholar]
- Schneider A, Ingram H. Social construction of target populations: Implications for politics and policy. The American Political Science Review. 1993;87:334–47. [Google Scholar]
- Shiffman J. A social explanation for the rise and fall of global health issues. Bulletin of the World Health Organization. 2009;87:608–13. doi: 10.2471/BLT.08.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman J, Quissell K, Schmitz HP, et al. A framework on the emergence and effectiveness of global health networks. Health Policy and Planning. 2016;31(Suppl. 1):3–16. doi: 10.1093/heapol/czu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman J, Schmitz HP, Berlan D, et al. The emergence and effectiveness of global health networks: findings and future research. Health Policy and Planning. 2016;31(Suppl. 1):110–123. doi: 10.1093/heapol/czw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkink K. The power of networks in international politics. In: Kahler M, editor. Networked Politics: Agency, Power, and Governance. Ithaca, NY: Cornell University Press; 2009. pp. 228–47. [Google Scholar]
- Smart T. Activist Chides TB Congress for Complacency in Facing Drug Resistance. 2006. TheBody.com. Available at: http://www.thebody.com/content/art39862.html, accessed 24 March 2012.
- Snow DA, Burke Rochford EB, Worden SK, et al. Frame alignment processes, micromobilization, and movement participation. American Sociological Review. 1986;51:464–81. [Google Scholar]
- Stone DA. Causal stories and the formation of policy agendas. Political Science Quarterly. 1989;104:281–300. [Google Scholar]
- Stop TB Partnership. Basic Framework for the Global Partnership to Stop TB. Geneva, Switzerland: The Stop TB Partnership; 2001. [Google Scholar]
- Stop TB Partnership. Stop TB Partnership Annual Report 2010. Geneva: Stop TB Partnership and WHO; 2010. [Google Scholar]
- Stop TB Partnership. 2014a. Global Drug Facility. http://www.stoptb.org/gdf/, accessed 18 January 2015.
- Stop TB Partnership. 2014b. The Co-ordinating Board. http://www.stoptb.org/about/cb/, accessed 18 January 2015.
- Stop TB Partnership. The Stop TB Partnership to move its Secretariat from WHO to UNOPS. 2014c. http://www.stoptb.org/news/stories/2014/ns14_049.asp, accessed 9 October 2014.
- Taskforce on Innovative International Financing for Health Systems. More Money for Health, and More Health for the Money. Geneva, Switzerland: International Health Partnership; 2009. [Google Scholar]
- The Global Fund. Annual Report 2002/2003. Geneva, Switzerland: The Global Fund to Fight AIDS, Tuberculosis, and Malaria; 2003. [Google Scholar]
- Walt G. Tuberculosis: an interdisciplinary perspective. London, UK: Imperial College Press; 1999. The politics of tuberculosis: The role of process and power; pp. 99–120. [Google Scholar]
- Walt G. International Public Health: Diseases, Programs, Systems, and Policies. London, UK: Jones & Bartlett Publishers; 2004. Global cooperation in international public health; pp. 667–99. [Google Scholar]
- Walt G, Buse K, Harmer A. Cooperation in global health. In: Merson M, Black R, Mills A, editors. Global Health: Diseases, Programs, Systems and Policies. Burlington, MA: Jones & Bartlett Learning; 2012. [Google Scholar]
- Weber T. Gates gives $600m more to stop TB. 2006. BBC. http://news.bbc.co.uk/2/hi/business/4653338.stm, accessed 21 February 2012.
- WHO. Global Tuberculosis Control Report 1998. Geneva: World Health Organization; 1998. [Google Scholar]
- WHO. First Stop TB Partners’ Forum. World Health Organization; 2001. [Google Scholar]
- WHO. Global Tuberculosis Control Report 2004. World Health Organization; 2004. [Google Scholar]
- WHO. Global Tuberculosis Control Report 2009. World Health Organization; 2009. [Google Scholar]
- WHO. 2010. Tuberculosis Fact Sheet. http://www.who.int/mediacentre/factsheets/fs104/en/index.html, accessed 2 February 2012.
- WHO. Global Tuberculosis Control Report 2011. World Health Organization; 2011. [Google Scholar]
- WHO. 2012a. Global Tuberculosis Control Report 2012.
- WHO. 2012b. WHO | Frequently asked questions about TB and HIV. WHO. http://www.who.int/tb/challenges/hiv/faq/en/, accessed 11 November 2012.
- WHO. 2012c. The Global TB Programme. http://www.who.int/tb/about/en/, accessed 6 November 2012.
- WHO. Global Tuberculosis Control Report 2013. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- WHO. Global Tuberculosis Control Report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- Woolcock M, Narayan D. Social capital: implications for development theory, research, and policy. The World Bank Research Observer. 2000;15:225–49. [Google Scholar]
- World Bank. 1993. World Development Report 1993: Investing in Health, Volume1. http://www.scribd.com/WorldBankPublications/d/16060155-World-Development-Report-1993-Investing-in-Health-Volume1, accessed 20 March 2012.
- Yin R. Case Study Research: Design and Methods. Thousand Oaks, CA: Sage Publications; 2008. [Google Scholar]
- Zwarenstein M, Schoeman JH, Vundule C, et al. Randomised controlled trial of self-supervised and directly observed treatment of tuberculosis. The Lancet. 1998;352:1340–3. doi: 10.1016/S0140-6736(98)04022-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.