ABSTRACT
The CFTR chloride channel is tightly regulated by phosphorylation at multiple serine residues. Recently it has been proposed that its activity is also regulated by tyrosine kinases, however the tyrosine phosphorylation sites remain to be identified. In this study we examined 2 candidate tyrosine residues near the boundary between the first nucleotide binding domain and the R domain, a region which is important for channel function but devoid of PKA consensus sequences. Mutating tyrosines at positions 625 and 627 dramatically reduced responses to Src or Pyk2 without altering the activation by PKA, suggesting they may contribute to CFTR regulation.
KEYWORDS: CFTR regulation, cystic fibrosis, phosphotyrosine, Pyk2, Src
Introduction
Cystic Fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel. Like other members of the ATP-binding cassette (ABC) transporter family, CFTR channel activity is regulated by the binding and hydrolysis of ATP molecules by the Nucleotide Binding Domains (NBDs).1,2 In addition, CFTR catalytic activity is initiated and regulated by phosphorylation of its unique, intrinsically disordered regulatory region or R-domain.
The phosphorylation state of CFTR is controlled by both kinases and phosphatases3 and many serines lie within consensus sequences for phosphorylation by PKA and PKC.4 Several studies have suggested that tyrosine kinases may also influence CFTR gating either directly or indirectly. Direct tyrosine kinase regulation was suggested by exposing excised membrane patches to c-Src kinase5 and by the sensitivity of muscarinic stimulation of CFTR to tyrosine kinase inhibitors.6 Indirect regulation through a mechanism in which tyrosine phosphorylation unmasks a potential Casein Kinase 2 (CK2) phospho-acceptor site (Ser511) in NBD1 has also been reported.7,8 We recently reported that that tyrosine phosphorylation can be a potent and direct (PKA/C-independent) stimulus for CFTR activation.9 Moreover, we found that the tyrosine kinases c-Src and Pyk2 are both able to phosphorylate CFTR on tyrosine residues, and activate chloride currents which are 80% as large as those stimulated by PKA.
The serine/threonine phosphorylation sites on CFTR have been identified10,11 and their contributions to channel regulation have been characterized in detail,12-14 however the tyrosine residues that mediate direct regulation of channel activity remain unknown. We have attempted to identify these sites using mass spectrometry but so far that approach has not been successful despite clear radiolabeling after incubation with Src and [γ-32P]-ATP and detection of a CFTR band in immunoprecipitates when immunoblots are probed with anti-phosphotyrosine antibody. In the present work, we used a site-directed mutagenesis/functional assay approach, focusing on 2 candidate tyrosine residues near the c-terminal end of NBD1 (Y625 and Y627). We found that substituting these 2 tyrosine residues with phenylalanine (CFTR-YY625,627FF mutant) dramatically reduced (but did not abolish) tyrosine kinase stimulated currents without altering those stimulated by PKA.
Results
None of the 40 tyrosine residues on CFTR are situated in canonical strong sites for phosphorylation by Src or other tyrosine kinases.15 As a first step toward localizing the critical tyrosines we examined the ability of Src to phosphorylate CFTR peptide fragments (Fig. 1). Polypeptides corresponding to full-length CFTR (a.a. 1-1480, lane 1and2), first membrane domain + NBD1 (a.a. 1-640, lane 3&4), first membrane domain + NBD1 + R domain (a.a. 1-830, lane 5&6) or all of the CFTR sequence that is distal to the fourth transmembrane segment (a.a. 281-1480, lane 7&8) were stably expressed in BHK cells. Peptides were immunoprecipitated from the control (−) or v-Src expressing (+) cells using a mouse monoclonal antibody against anti-phosphotyrosine, and immunoblots were probed with the anti-CFTR antibody L12B4. When co-expressed with the consitutively active tyrosine kinase v-Src, all CFTR peptides could be immunoprecipitated by anti-phosphotyrosine antibody as indicated by bands at their predicted molecular weights (Fig. 1 black arrows). Tyrosine phosphorylation of all 3 peptides might be explained by the phosphorylation of one or more tyrosines between a.a. 281-640, the region of CFTR which is shared by these peptides, which we refer to as the “optimistic hypothesis”. Alternatively, the results are also consistent with the presence of multiple phosphotyrosines distributed throughout the molecule.
Figure 1.
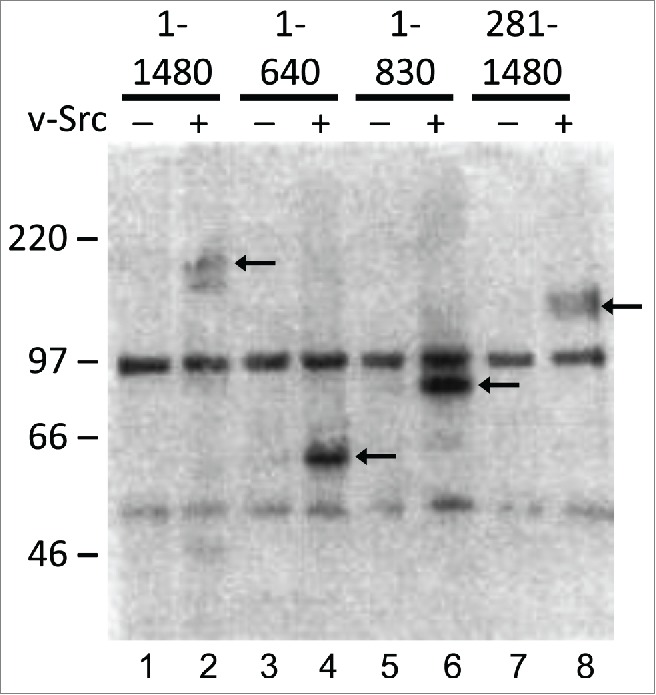
Phosphorylation of CFTR by Src kinase. Blot of anto-phosphotyrosine immunoprecipitates probed with the anti-CFTR monoclonal antibody L12B4. CFTR peptides (identified by the boundary amino acid numbers indicated above each pair of lanes) co-expressed with or without v-Src and precipitated using monoclonal antibody against phosphotyrosine. Black arrows indicate the positions of CFTR phosphopeptides. The bands at ˜50 and ˜100 kDa observed in every lane indicate mono- and dimeric heavy chains of the immunoprecipitating IgG, which are recognized by the secondary antibody used for immunoblotting. Black arrows indicate CFTR fragments. A representative of 2 experiments is shown.
We tested the optimistic hypothesis (i.e., that phosphotyrosine is detectable on all the peptides because it is situated in the region of overlap) by substituting the most likely candidate tyrosine residues, Y625 and Y627, simultaneously with phenylalanine. Silent patches were excised from BHK stably expressing the double mutant (CFTR-YY625,627FF) and exposed to active phospho-Src or to the catalytic domain of Pyk2 (30U/ml for both kinases). Wt-CFTR current was strongly activated by both tyrosine kinases. Figure 2A and B show representative traces of wt-CFTR activity induced by Src and Pyk2, respectively. By contrast, CFTR channel activation by the 2 tested tyrosine kinases was strongly decreased in the double mutant compared to the normal responses to PKA (75 nM), which was added at the end of each experiment (Fig. 2C and D). Strong activation by PKA confirmed the presence of mutant CFTR channels in the patch and indicate that substituting phenylalanine at Y625 and Y627 did not grossly affect channel function.
Figure 2.
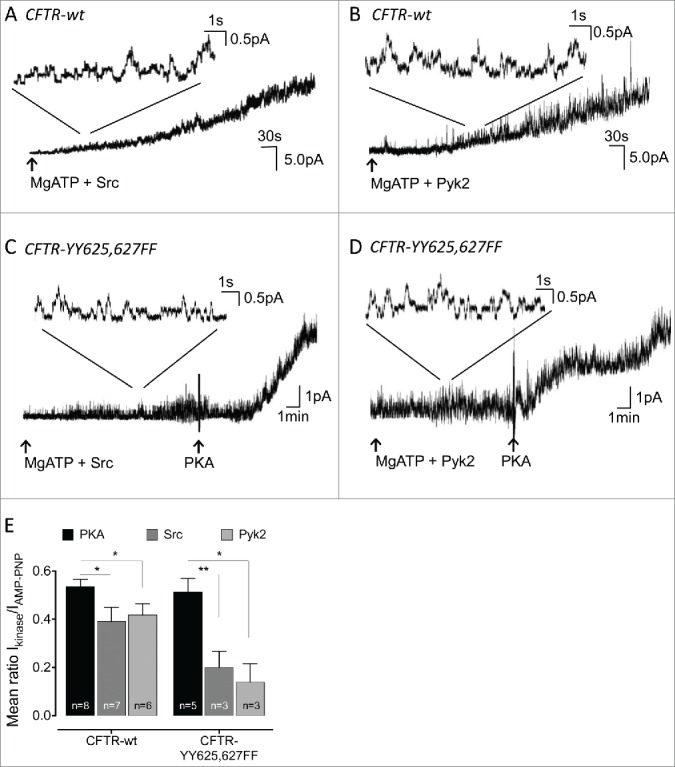
Effect of Src and Pyk2 on activity of the CFTR double mutant YY625,627FF. (A–D): Effect of Src (A & C) and Pyk2 (B & D) on channel activity in patch excised from cell expressing wt (A&B) or YY625,627FF (C & D) CFTR. (E): Comparison of the mean ratio of the kinase- stimulated currents measured before and after addition of 1 mM AMP-PNP. Error bars show SE for number of cells indicated. *, p < 0.05; **, p < 0.01 with student t-test. The wt-CFTR results from Billet et al 20159 obtained under identical conditions are reproduced here for comparison with YY625,627FF-CFTR mutant.
To compare the activation by different kinases we added the non-hydrolysable ATP analog AMP-PNP (1mM) at the end of experiments and calculated the current ratio to help normalize for variation in the number of channels per patch as described previously.9 Figure 2E shows a histogram of the current ratios obtained in presence of 30U/ml Src, Pyk2, or 75nM PKA. The ratios revealed a dramatically decrease in the activation of YY625,627FF CFTR by Src or Pyk2 (current ratios 0.20 and 0.13 respectively, n = 3) compared to wild-type CFTR. This contrasted with the current ratio during application of PKA, which was similar for mutant (current ratio 0.51, n = 5) and wild-type channels. These data strongly suggest that phosphorylation of one or both tyrosines contributes to CFTR activation by Src and Pyk2, although part (25 – 40%) of the response is apparently due to other sites which remain to be identified.
Discussion
In a previous study we found that the tyrosine kinases Src and Pyk2 can activate wild-type CFTR, producing currents that reach 80% of those stimulated by PKA.9 Here we observed a dramatic decrease in tyrosine kinase-activated currents when Y625 and Y627 were mutagenized to phenylalanine (YY625,627FF) in full-length CFTR. Since PKA stimulation was not altered in the double mutant, one or both tyrosine residues may contribute to CFTR activation by tyrosine kinases.
As a first approach toward localizing the site we coexpressed peptides corresponding to different regions of CFTR together with v-Src and examined their in vivo phosphorylation by immunoprecipitation with anti-phosphotyrosine antibody and blotting with anti-CFTR antibody. Phosphotyrosine was detected on all 3 peptides, therefore we focused on tyrosines in the region of overlap (amino acids 281 – 640), which occur in the sequence KILI[LHEGSSY625]FY627GTF.
Based on the preferential phosphorylation of sequences in a peptide library, E(2-3)-I/V-Y- G/E-X-F15 was proposed to be the ideal Src consensus. The three residues following Y627 fit this consensus, however the non-polar residue and 2 or 3 upstream glutamates are lacking. This divergence from the ideal consensus may explain why high activity, i.e., heterologous expression of constitutively active v-Src, was needed to be able to detect tyrosine phosphorylation in intact cells. We cannot exclude other explanations however as the specificity may depend on factors other than the primary sequence, and Src kinases are known to phosphorylate a range of target sequences.16 The sequence upstream of Y625 and Y627 bears some resemblance to the generic protein tyrosine kinase (PTK) consensus R/K-[X(2/3)-D/E-X(3/2)-Y], suggesting these sites in CFTR might also be a substrates for other tyrosine kinases under physiological conditions. The lack of PKA sites near these sites reinforce the notion that phosphotyrosine acts specifically rather than as a surrogate for phosphoserine.
Interestingly, Y625 and Y627 are situated at the junction between NBD1 and the R domain, a region in which structural integrity is crucial for channel regulation and pharmacology.17,18 Further studies of tyrosine kinase regulation may provide a better understanding of R domain structure and the conformational changes associated with CFTR channel regulation.
Methods
Plasmid and cell culture
Baby hamster kidney (BHK) cells stably expressing wild-type CFTR or the YY625,627FF-CFTR mutant10 were plated on plastic coverslips at low density and cultured in MEM (Life Technologies, Burlington, ON, Canada) containing 5% FBS and the selecting drug methotrexate (300-500 µM) for 2 days with 5% CO2 at 37°C. To express peptides comprising different regions of CFTR, cDNAs encoding amino acids 1-640, 1-830, and 281- 1480 were prepared by PCR mutagenesis, inserted into the pNUT vector, and stably transfected into BHK cells. The role of tyrosines 625 and 627 of CFTR in the activation by Src was examined by substituting them with phenylalanines using site-directed mutagenesis. An expression plasmid containing v-Src cDNA was kindly provided by Dr. R. Huganir (Department of Neuroscience, Johns Hopkins University) and co-transfected with large T antigen plasmid into BHK cells using calcium phosphate co-precipitation.
Tyrosine phosphorylation
To detect phosphotyrosine on CFTR, cells in 25 cm2 dishes were transiently transfected using calcium phosphate coprecipitation with 5 μg of v-Src plasmid DNA and 1 μg of a large T-antigen expressing plasmid. Two days after Src transfection, cells were lysed in RIPA buffer and subjected to sequential immunoprecipitation and immunoblotting. For immunoprecipitation we used an anti-phosphotyrosine monoclonal antibody, which recognizes an epitope between residues 1365 and 1395 of CFTR. The monoclonal antibody L12B4, which recognizes an epitope between residues 386 and 412 of CFTR, was used for immunoblotting.
Patch clamp
Microscopic CFTR currents were recorded from inside-out patches as previously described.9 The ratio of currents activated by kinase in the absence vs presence of AMP-PNP (which further increases open probability to near Po = 1.0) allowed CFTR responses to be normalized for variations in the number of channels per patch. Recombinant Src (30U/ml) was from EMD Millipore; the catalytic domain of Pyk2(30U/ml) was from Cedarlane (Burlington, ON, Canada); PKA (final concentration, 75 nM–30 U) was from Promega (Madison, WI, USA); MgATP (1 mM) was from Sigma-Aldrich, and AMP-PNP (1 mM) was from Sigma-Aldrich (St. Louis, MO, USA).
Acknowledgments
AB was supported by fellowships from the Groupe d’Étude des Protéines Membranaire (GÉPROM), the Groupe de Recherche Axé sur la Structure des Protéines (GRASP), the McGill CIHR Program in Chemical Biology and the McGill Faculty of Medicine. YJ was supported by the Respiratory Health Network of Centers of Excellence and fellowships from the Canadian Lung Association/Medical Research Council and Canadian Cystic Fibrosis Foundation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by grants from the NIH and CIHR to JRR and JWH, respectively.
References
- [1].Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J Biol Chem 2002; 277:15419-25; PMID:11861646; http://dx.doi.org/ 10.1074/jbc.M111713200 [DOI] [PubMed] [Google Scholar]
- [2].Basso C, Vergani P, Nairn AC, Gadsby DC. Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating. J Gen Physiol 2003; 122:333-48; PMID:12939393; http://dx.doi.org/ 10.1085/jgp.200308798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wilkinson DJ, Strong T V, Mansoura MK, Wood DL, Smith SS, Collins FS, Dawson DC. CFTR activation: additive effects of stimulatory and inhibitory phosphorylation sites in the R domain. Am J Physiol 1997; 273:L127-33; PMID:9252549 [DOI] [PubMed] [Google Scholar]
- [4].Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev 1999; 79:S77-107; PMID:9922377 [DOI] [PubMed] [Google Scholar]
- [5].Fischer H, Machen TE. The tyrosine kinase p60c-src regulates the fast gate of the cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J 1996; 71:3073-82; PMID:8968578; http://dx.doi.org/ 10.1016/S0006-3495(96)79501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Billet A, Luo Y, Balghi H, Hanrahan JW. Role of Tyrosine Phosphorylation in the Muscarinic Activation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). J Biol Chem 2013; 288:21815-23; PMID: 23760269; http://dx.doi.org/ 10.1074/jbc.M113.479360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cesaro L, Marin O, Venerando A, Donella-Deana A, Pinna LA. Phosphorylation of cystic fibrosis transmembrane conductance regulator (CFTR) serine-511 by the combined action of tyrosine kinases and CK2: the implication of tyrosine-512 and phenylalanine-508. Amino Acids 2013; 45:1423-9; PMID:24178769; http://dx.doi.org/ 10.1007/s00726-013-1613-y [DOI] [PubMed] [Google Scholar]
- [8].Venerando A, Cesaro L, Marin O, Donella-Deana A, Pinna LA. A “SYDE” effect of hierarchical phosphorylation: possible relevance to the cystic fibrosis basic defect. Cell Mol Life Sci 2014; 71:2193-6; PMID:24566881; http://dx.doi.org/ 10.1007/s00018-014-1581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Billet A, Jia Y, Jensen T, Riordan JR, Hanrahan JW. Regulation of the cystic fibrosis transmembrane conductance regulator anion channel by tyrosine phosphorylation. FASEB J 2015; 29:3945-53; PMID:26062600; http://dx.doi.org/ 10.1096/fj.15-273151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seibert FS, Chang XB, Aleksandrov AA, Clarke DM, Hanrahan JW, Riordan JR. Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum. Biochim Biophys Acta 1999; 1461:275-83; PMID:10581361; http://dx.doi.org/ 10.1016/S0005-2736(99)00163-7 [DOI] [PubMed] [Google Scholar]
- [11].Chappe V, Hinkson DA, Zhu T, Chang X-B, Riordan JR, Hanrahan JW. Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol 2003; 548:39-52; PMID:12588899; http://dx.doi.org/ 10.1113/jphysiol.2002.035790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vais H, Zhang R, Reenstra WW. Dibasic phosphorylation sites in the R domain of CFTR have stimulatory and inhibitory effects on channel activation. Am J Physiol Cell Physiol 2004; 287:C737-45; PMID:15140750; http://dx.doi.org/ 10.1152/ajpcell.00504.2003 [DOI] [PubMed] [Google Scholar]
- [13].Csanády L, Seto-Young D, Chan KW, Cenciarelli C, Angel BB, Qin J, McLachlin DT, Krutchinsky AN, Chait BT, Nairn AC, et al.. Preferential phosphorylation of R-domain Serine 768 dampens activation of CFTR channels by PKA. J Gen Physiol 2005; 125:171-86; http://dx.doi.org/ 10.1085/jgp.200409076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chappe V, Hinkson DA, Howell LD, Evagelidis A, Liao J, Chang X-B, Riordan JR, Hanrahan JW. Stimulatory and inhibitory protein kinase C consensus sequences regulate the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A 2004; 101:390-5; PMID:14695900; http://dx.doi.org/ 10.1073/pnas.0303411101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Songyang Z, Carraway KL, Eck MJ, Harrison SC, Feldman RA, Mohammadi M, Schlessinger J, Hubbard SR, Smith DP, Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 1995; 373:536-9; PMID:7845468; http://dx.doi.org/ 10.1038/373536a0 [DOI] [PubMed] [Google Scholar]
- [16].Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol 1991; 200:62-81; PMID:1956339; http://dx.doi.org/ 10.1016/0076-6879(91)00127-I [DOI] [PubMed] [Google Scholar]
- [17].Billet A, Melin P, Jollivet M, Mornon J-P, Callebaut I, Becq F. C terminus of nucleotide binding domain 1 contains critical features for cystic fibrosis transmembrane conductance regulator trafficking and activation. J Biol Chem 2010; 285:22132-40; PMID:20435887; http://dx.doi.org/ 10.1074/jbc.M110.120683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vankeerberghen A, Wei L, Jaspers M, Cassiman JJ, Nilius B, Cuppens H. Characterization of 19 disease-associated missense mutations in the regulatory domain of the cystic fibrosis transmembrane conductance regulator. Hum Mol Genet 1998; 7:1761-9; PMID:9736778; http://dx.doi.org/ 10.1093/hmg/7.11.1761 [DOI] [PubMed] [Google Scholar]


