ABSTRACT
Voltage-gated calcium (CaV) channels are responsible for Ca2+ influx in excitable cells. As one of the auxiliary subunits, the CaV β subunit plays a pivotal role in the membrane expression and receptor modulation of CaV channels. In particular, the subcellular localization of the β subunit is critical for determining the biophysical properties of CaV channels. Recently, we showed that the β2e isotype is tethered to the plasma membrane. Such a feature of β2e is due to the reversible electrostatic interaction with anionic membrane phospholipids. Here, we further explored the membrane interaction property of β2e by comparing it with that of myristoylated alanine-rich C kinase substrate (MARCKS). First, the charge neutralization of the inner leaf of the plasma membrane induced the translocation of both β2e and MARCKS to the cytosol, while the transient depletion of poly-phosphoinositides (poly-PIs) by translocatable pseudojanin (PJ) systems induced the cytosolic translocation of β2e but not MARCKS. Second, the activation of protein kinase C (PKC) induced the translocation of MARCKS but not β2e. We also found that after the cytosolic translocation of MARCKS by receptor activation, depletion of poly-PIs slowed the recovery of MARCKS to the plasma membrane. Together, our data demonstrate that both β2e and MARCKS bind to the membrane through electrostatic interaction but with different binding affinity, and thus, they are differentially regulated by enzymatic degradation of membrane PIs.
KEYWORDS: electrostatic interaction; myristoylated alanine-rich C kinase substrate (MARCKS); phosphatidylinositol 4,5-bisphosphate (PIP2); protein kinase C (PKC); voltage-gated calcium channel; β2e subunit
Introduction
Voltage-gated calcium (CaV) channels govern Ca2+ entry in response to the depolarization of membrane potential in excitable cells.1 Ca2+ influx relays the diverse neuronal processes, including neurotransmitter and hormone release and gene transcription.2,3 A high-voltage-activated CaV channel is composed of at least α1, β, and α2δ subunits. Although the α1 subunit primarily determines the voltage-sensitivity and pharmacological responses of CaV channels, as auxiliary subunits, β and α2δ subunits also play a pivotal role in regulating CaV channel activity and promoting their membrane expression.4 In particular, β subunits have a strong influence on the biophysical property of CaV channel gating.5,6 With 4 isotypes, β subunits are usually located in the cytosol and bind to the CaV channel via the α-interacting domain. However, β2a and β2e subunits are tethered to the plasma membrane even in the absence of α1.7,8 Whether β subunits are localized in the cytosol or in the plasma membrane is a key determinant particularly in terms of inactivation kinetics and modulation by membrane phospholipids. Whereas CaV channels with cytosolic β subunits show fast current inactivation and high sensitivity to the depletion of phosphatidylinositol 4,5-bisphosphate (PIP2), channels with membrane-anchored β subunits exhibit the opposite responses.9,10 Recent studies showed that the β2e subunit is associated with the plasma membrane through electrostatic interaction, while β2a is tethered to the plasma membrane through palmitoylation in the N-terminus.11-13 In particular, membrane phosphoinositides (PIs) are involved in the association with several basic amino acid residues in the N-terminus of the β2e subunit, leading to the possibility that the location of the β2e subunit can be changed by membrane PI dynamics.
For the membrane association of peripheral membrane proteins, PIs in the inner leaflet of the plasma membrane function as acceptors for protein tethering in various cellular signaling pathways.14,15 The merit of membrane recruitment of peripheral membrane proteins via anionic PIs is that it allows reversible binding in response to their depletion and resynthesis.16,17 Hence, the electrostatic interaction between proteins and membrane lipids plays a critical role in the regulation of physiological processes. Our recent works showed that the β2e subunit is liberated from the plasma membrane when both PIP2 and phosphatidylinositol 4-phosphate (PI4P) are simultaneously depleted by rapamycin-inducible dimerization systems.13 In addition, the stimulation of Gq-coupled receptors with agonists induced β2e translocation to the cytosol through the depletion of poly-PIs by PLC activation. The molecular dynamics (MD) simulation suggests that the β2e subunit interacts with the plasma membrane in a stretched binding mode, where the N-terminal Lys-2 (K2) and Trp-5 (W5) proximal residues are important for the stable membrane association of β2e, as they unfold the N-terminal structure.12 This binding mode seems to be analogous to the membrane binding of myristoylated alanine-rich C kinase substrate (MARCKS), a well-known protein that associates with the plasma membrane through non-specific electrostatic interaction.18,19 Here, we further investigated the membrane-tethering property of β2e by comparing it with that of MARCKS. Our data demonstrate that both β2e and MARCKS electrostatically interact with anionic phospholipids but with different affinity, and thus, they are differentially regulated by receptor-mediated signaling.
Results
Both β2e and MARCKS are tethered to the plasma membrane through electrostatic interaction
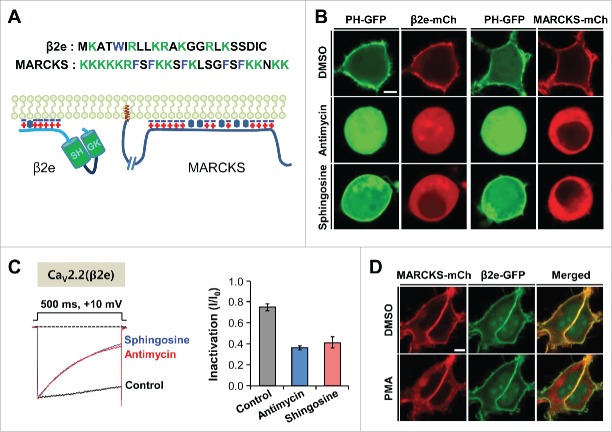
Using diverse approaches, we recently showed that the β2e subunit is associated with the plasma membrane through electrostatic interaction between the β2e N-terminus and anionic membrane phospholipids.12,13 As shown in Figure 1A and B, both β2e and MARCKS consistently have groups of basic residues and hydrophobic residue(s) and are localized in the plasma membrane in the exogenously expressed cells. We first examined whether the reduction of membrane electric charges induces changes in their subcellular distributions. For this, we used antimycin and sphingosine, which diminish membrane anionic charges. When antimycin was applied to decrease the charges through the depletion of membrane PIs,20 both β2e and MARCKS exhibited cytosolic distributions. Likewise, the neutralization of the negative charges of membrane phospholipids by the addition of sphingosine, which has a +1 charge, induced their cytosolic localization (Fig. 1B).21 Furthermore, both drugs promoted current inactivation in CaV2.2 channels with β2e (Fig. 1C). Those results suggest that disruption of the charge–charge interaction between β2e and membrane phospholipids resulted in the dissociation of β2e from the plasma membrane and induced fast inactivation of the CaV current, which is consistent with previous reports indicating that the membrane tethering of β subunits in cells is important in regulating CaV channel gating.10,22 It has been shown that MARCKS is a substrate of protein kinase C (PKC) and that phosphorylation of the proteins by PKC causes the translocation from the plasma membrane to the cytosol.23 When cells expressing both MARCKS and β2e were applied with the PKC activator phorbol 12-myristate 13-acetate (PMA), MARCKS, but not β2e, was strongly translocated to the cytosol (Fig. 1D), suggesting that β2e location is insensitive to PKC activation.
Figure 1.
Membrane tethering of β2e and MARCKS was antagonized by the reduction of negative charges in the inner leaflet of the plasma membrane. (A) Sequences of N-terminal region of β2e and effector domain of MARCKS (Top) and schematic representations of membrane-bound β2e and MARCKS (Bottom). Blue minus signs and red plus signs indicate acidic lipids and basic amino acid residues, respectively. Hydrophobic residues and myristoylation are represented by hexagons and brown colored signs (MARCKS), respectively. (B) Confocal images of cells expressing PH-PLCδ-GFP (PH-GFP) and β2e-mCh (mCherry) or PH-GFP and MARCKS-mCh in response to 200 nM antimycin/10 mM deoxyglucose or 75 µM sphingosine. The cells were pretreated with the drugs for 40 min. Bar, 5 μm. (C) Effect of antimycin and sphingosine on current inactivation of CaV2.2 channels. Currents were recorded during 500-ms test pulses to +10 mV. Dashed line indicates the zero current. For analysis, n = 5-6. (D) Confocal images of cells co-expressing MARCKS-mCh and β2e-GFP in response to 500 nM PMA for 2 min. Bar, 5 μm.
Depletion of poly-PIs induces cytosolic translocation of β2e but not MARCKS
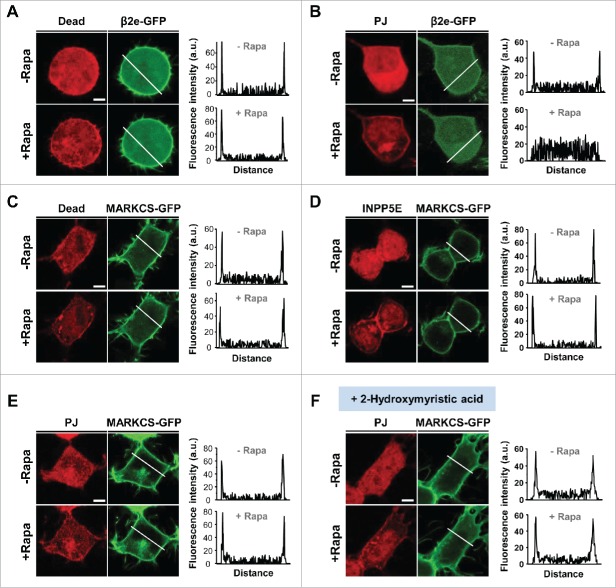
Since both β2e and MARCKS are tethered to the plasma membrane via the coupling with phospholipids, using rapamycin-inducible dimerization systems, we examined whether depletion of poly-PIs changes the location of the proteins. Our data showed that rapamycin-induced membrane recruitment of pseudojanin (PJ), which consists of PtdIns 4-phosphatase (Sac) and PIP2 5-phosphatase (INPP5E),24 led to the dissociation of β2e from the plasma membrane (Fig. 2A and B). When this tool was used for MARCKS, unlike the behavior of β2e, membrane recruitment of INPP5E or PJ did not influence the localization of MARCKS (Fig. 2C, D, and E). Considering that N-terminal myristoylation of MARCKS is partially involved in membrane binding,25 such lipidation may prevent membrane dissociation of the protein from enzymatic activity. However, in cells treated with myristoylation inhibitor 2-hydroxymyristic acid (2-HM), the parallel experiments performed under the same condition showed that de-myristoylation did not affect the location of MARCKS (Fig. 2F), suggesting that MARCKS interaction with the plasma membrane is independent from the lipidation and insensitive to enzymatic activity for degradation of membrane PIs.
Figure 2.
Effect of PI4P and PIP2 on plasma membrane tethering of β2e and MARCKS. (A) (B) Confocal images and line scanning of cells expressing inactive (Dead) or active form of pseudojanin (PJ) with LDR and β2e-GFP before and after 1 μM rapamycin for 2 min. White lines in images represent line scanned regions. Bar, 5 μm. (C) (D) (E) Confocal images and line scanning of cells expressing Dead, PIP2 5-phosphatase (INPP5E) or PJ with LDR and MARCKS. Bar, 5 μm. (F) Effect of PJ translocation on MARCKS distribution in cells treated with 100 μM of 2-Hydroxymyristic acid for 2 min. Bar, 5 μm.
We further investigated the functional role of PI4P and PIP2 in the membrane association of MARCKS. It is well known that cross-talk between MARCKS and signaling proteins, such as PKC and calmodulin, is important in determining MARCKS location.26 The tsA201 cells used in the current study expressed various Gq-coupled receptors (GPCRs).28 We hypothesized that stimulation of GPCRs may trigger the cytosolic translocation of MARCKS through the activation of PKC. Indeed, the addition of ATP induced the dissociation of MARCKS from the plasma membrane through the activation of endogenous purinergic receptors.29 Next, we examined whether the membrane recruitment of MARCKS could be delayed when the poly-PIs were depleted. As shown in Figure 3A and B, after the cytosolic translocation of MARCKS by ATP treatment, the removal of PI4P and PIP2 by translocatable PJ systems slowed down the recovery of MARCKS to the plasma membrane. The results suggest the possibility that MARCKS interacts with membrane poly-PIs very tightly and thus the coupling is resistant to PI degradation by lipid phosphatases.
Figure 3.
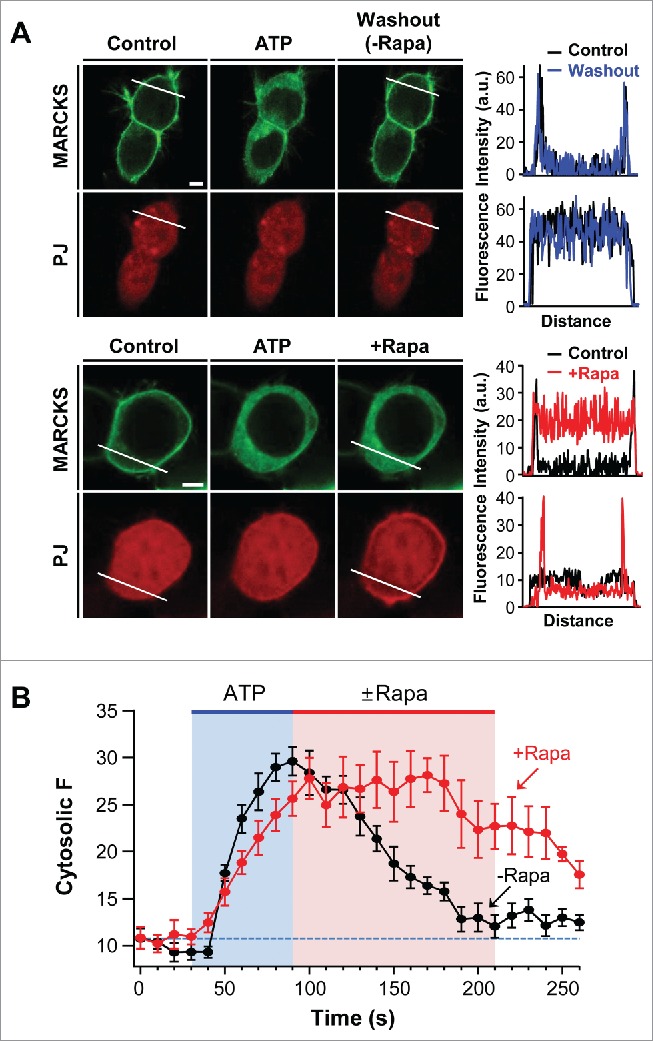
Depletion of PI4P and PIP2 delays recovery of MARCKS to the plasma membrane. (A) Confocal images and line scanning of cells expressing LDR, PJ, and MARCKS-GFP in response to 50 μM ATP and 1 μM rapamycin. Top and bottom panels represent location change of MARCKS in the absence (black) and presence (blue) of rapamycin, respectively. Bar, 5 μm. (B) Time course of MARCKS translocation in response to the absence or presence of rapamycin. Images of time courses were taken every 5 s by confocal microscope. For analysis, n = 4 .
PIP3 depletion has no effect on the membrane tethering of β2e or MARCKS
PIP3 also contributes negativity to the inner leaflet of the plasma membrane. To determine whether PIP3 affects the cellular location of β2e and MARCKS, we utilized an engineered 3-phosphatase enzyme CF-PTEN and a dominant negative regulatory form of PI3K (Δp85) to deplete PIP3 in live cells.30,31 Recently, we generated a chimeric protein, CF-PTEN, which specifically depletes PIP3 through rapamycin-inducible dimerization. First, in cells expressing Btk-PH-GFP (Btk-GFP) as a PIP3 probe, the membrane recruitment of CF-PTEN by the addition of rapamycin triggered the cytosolic movement of Btk-GFP from the plasma membrane (Fig. 4A). In contrast, the depletion of PIP3 had no influence on the location of β2e or MARCKS, suggesting that the translocation of β2e and MARCKS requires combinatory play of membrane PIs (Fig. 4B). In the analysis of current inactivation, the depletion of PIP3 did not affect inactivation of the CaV2.2 channel with β2e (Fig. 4C). In addition to the depletion of PIP3, we also examined the cellular distribution of β2e and MARCKS after reducing PI3K activity, because PIP3 may involve the initial membrane recruitment of MARCKS. To test thisit, we made use of Δp85, which is a dominant-negative form of the PI3K regulatory subunit.31 In cells expressing both Btk-GFP and β2e-mCh, while the Btk-GFP probe was expressed in the plasma membrane, it displayed cytosolic expression in the cells expressing Δp85. On the other hand, β2e was expressed in the plasma membrane, suggesting that membrane targeting of β2e is insensitive to PIP3. In the experiment with MARCKS, the decrease of PIP3 had no influence on the subcellular distribution of MARCKS. Collectively, these results indicate that membrane PIP3 is insufficient for the membrane binding of β2e and MARCKS.
Figure 4.
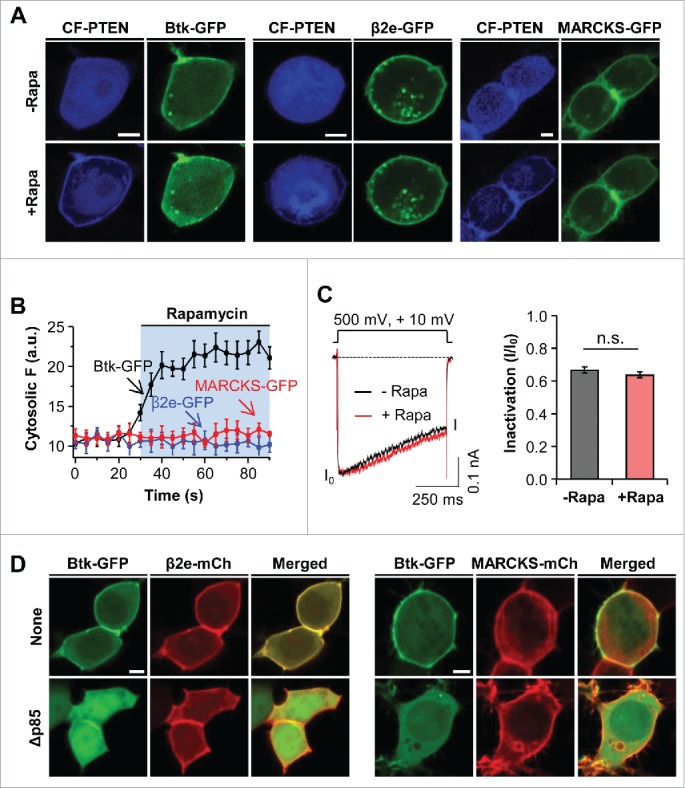
Effect of PIP3 on subcellular localization of β2e and MARCKS. (A) Confocal images of cells expressing LDR and CF-PTEN with Btk-PH, β2e-GFP, or MARCKS-GFP before and after the addition of 1 μM rapamycin for 2 mins. (B) Time courses of the effects of PIP3 depletion on cytosolic translocation of Btk-PH, β2e-GFP, and MARCKS-GFP. For analysis, n=4. (C) Current inactivation of CaV2.2 channels upon PIP3 depletion. Currents were measured during a 500-ms test pulse to +10 mV. For quantification, n=5. (D) Confocal images of cells expressing Btk-GFP with β2e-mCh or MARCKS-mCh in the absence or presence of dominant-negative p85 (Δp85). Bar, 5 μm.
Discussion
We recently found that the subcellular distribution of β2e is altered by the dynamic regulation of PIs and that such a location change of β2e has a significant influence on the gating property of CaV channels.12,13 Moreover, CaV channels with cytosolic mutant forms of β2e (K2A and W5A) exhibit fast inactivation and high PIP2 dependence.13 Here, our results extend our understanding of the interaction attribute of β2e with the plasma membrane by comparing it with that of MARCKS.
Firstly, with pharmacological agents, we confirmed that membrane charges are needed for the membrane localization of β2e and MARCKS. As a membrane-permeant base, sphingosine is generally used for neutralizing the electrostatic charge of membrane phospholipids, thus interfering with the interaction between these phospholipids and positively charged molecules.32 In the case of antimycin, it is known that its inhibitory effect on ATP synthesis blocks the synthesis of poly-PIs (e.g., PI4P, PIP2, and PIP3), resulting in a reduction of membrane negative charges.21 In our data, when each drug was applied to cells expressing β2e or MARCKS, the proteins were located in the cytosol, suggesting that both β2e and MARCKS bind to the plasma membrane through similar electrostatic interactions. From a structural viewpoint, MARCKS is a globular and elongated form belonging to the class of natively unfolded or unstructured proteins. It associates with the plasma membrane via an effector domain consisting of a group of basic residues and 5 phenylalanine residues.26 The fact that it is unfolded provides it the structural flexibility for binding to diverse ligands, such as membrane PIs and PS.27 Likewise, MD simulation analysis of β2e revealed that the N-terminal region of β2e associates with membrane phospholipids through a stretched binding mode.12 Further, it showed that the proximal Lys and Trp residues within the N-terminus are critical sites for the strong and stable membrane binding of β2e. Collectively, these results indicated that both β2e and MARCKS, with a structural similarity, interact with the plasma membrane through nonspecific electrostatic interactions.
For the dissociation of MARCKS from the plasma membrane, it has been shown that 3 serine residues in the effector domain of MARCKS can be phosphorylated by PKC, resulting in the translocation of MARCKS from the membrane to the cytosol due to the reduction of electrostatic interaction with anionic lipids.33 A previous study suggested a possibility that as with MARCKS, the 3 Ser/Thr residues in the N-terminal region of β2e could be target sites for phosphorylation by PKC and phosphorylation of the residues may induce dissociation of the subunit from the plasma membrane.11 However, with a PKC activator, our data revealed that PKC activation had no effect on the location change of β2e, indicating that membrane localization of the β2e subunit mainly depends on anionic membrane phospholipids. Even though extensive tests are needed to demonstrate the possible phosphorylation of β2e, this result shows that unlike MARCKS, the activation of PKC has no influence on the location change of β2e.
From the perspective of membrane lipids, our previous data showed that the depletion of both PI4P and PIP2 by rapamycin-inducible PJ systems or M1 muscarinic stimulation induced the translocation of β2e from the plasma membrane to the cytosol.13 In addition to PIs, a peptide-to-liposome FRET assay revealed that phosphatidylserine (PS) also plays an important role in the membrane binding of β2e,12 suggesting that various anionic membrane phospholipids could be involved in the membrane recruitment of β2e. Nevertheless, in the present study, enzymatic removal of PI4P and PIP2 failed to dissociate MARCKS from the plasma membrane, even in the absence of myristoylation in the N-terminal cysteine residue of MARCKS. However, when PIP2 and PIP were depleted by lipid phosphatase systems, the recovery of MARCKS to the plasma membrane was inhibited. These results suggest that MARCKS still binds to poly-PIs in the plasma membrane and possibly protects them from degradation by rapamycin-inducible PJ systems. Indeed, these findings are in line with previous reports indicating that the membrane expression of MARCKS prevents PIP2 depletion from PLC-mediated hydrolysis, indicating that MARCKS serves as a strong PIP2 sequester.34 Although more quantitative approaches are needed to compare the molecular properties of the 2 proteins in membrane binding, β2e appears to be more easily liberated from the plasma membrane under the condition of membrane phospholipid depletion. Thus, the mechanism by which the dynamic location of β2e is changed by membrane phospholipid alteration provides a simple but effective strategy for regulating the gating property of CaV channels.
In conclusion, our data show that screening of the anionic inner leaflet of the plasma membrane or inhibition of phospholipid synthesis induces the translocation of β2e from the plasma membrane to the cytosol and thus the fast inactivation of CaV channels. In addition, the results suggest that compared to MARCKS, β2e is relatively sensitive to turnover of membrane poly-PIs, including PI4P and PIP2. Considering the pivotal role of CaV channels in neurons, a regulatory mechanism of CaV channels by β2e could be extensively applicable in various neuronal systems.
Materials and methods
Cell culture and cDNA constructs
For patch clamp and confocal images, tsA201 cells were cultured and transiently transfected using Lipofectamine 2000 (Invitrogen) as previously described.13 MARCKS (NM_002356.5) was donated by Dr. Hoguen Kim from Yonsei University College of Medicine (Seoul, Korea), and dominant-negative P85 (Δp85) was donated by Ken Mackie from Indiana University (Bloomington, IN). For MARCKS-GFP and MARCKS-mCherry fusion proteins, MARCKS was amplified by PCR using the following primers: forward primer: 5′-ATGGGTGCCAGTTCTCCAAGACCGCA-3′; reverse primer: 5′-GGATCCCGCTCGGCCGTTGGCGCGGGG-3′. The PCR product was subcloned into pGEM-T Easy Vector (Promega) and then inserted into pEGFP-N1 and mCherry-N1 (Clontech) using ApaI and BamHI.
Patch clamping
Whole-cell configuration patch clamps were performed as previously described.13
Confocal imaging
Confocal imaging was performed by LSM700 (Carl Zeiss), and confocal images were processed using ZEN 2012 Lite Imaging Software as previously described.13
Chemicals
Antimycin (Sigma) and sphingosine (Sigma) were prepared as 1-mM and 75-mM stock solutions in DMSO. Working solutions, were prepared by diluting the stock at 1:5000 and 1:1000 with Ringer's solution, respectively. PMA (Enzo) was stocked as 500 µM in DMSO and prepared by diluting the stock at 1:1000. ATP (Sigma) was dissolved in dH2O to make a 50-mM stock solution and was diluted at 1:1,000 in Ringer's solution.
Acknowledgments
We thank all laboratories for providing the plasmids.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the DGIST R&D Program of the Ministry of Science, ICT& Future Planning (no. 16-BD-06).
References
- [1].Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 2000; 16:521–55; PMID:11031246; http://dx.doi.org/ 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- [2].Clapham DE. Calcium signaling. Cell 2008; 131:1047–58; http://dx.doi.org/ 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- [3].Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol 2000; 1:11–21; PMID:11413485; http://dx.doi.org/ 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- [4].Simms BA, Zamponi GW. Trafficking and stability of voltage-gated calcium channels. Cell Mol Life Sci 2012; 69:843–56; PMID:21964928; http://dx.doi.org/ 10.1007/s00018-011-0843-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buraei Z, Yang Y. The β subunit of voltage-gated Ca2+ channels. Physiol Rev 2013; 90:1461–506; http://dx.doi.org/ 10.1152/physrev.00057.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buraei Z, Yang J. Structure and function of the β subunit of voltage-gated Ca2 channels. Biochim Biophys Acta 2013; 1828:1530–40; PMID:22981275; http://dx.doi.org/ 10.1016/j.bbamem.2012.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel β2a subunit and effects on channel function. J Biol Chem 1996; 271:26465–8; PMID:8900112; http://dx.doi.org/ 10.1074/jbc.271.43.26465 [DOI] [PubMed] [Google Scholar]
- [8].Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effect of five human auxiliary β2 subunit splice variants on L-type calcium channel gating. Biophys J 2003; 84:3007–21; PMID:12719232; http://dx.doi.org/ 10.1016/S0006-3495(03)70027-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heneghan JF, Mitra-Ganguli T, Stanish LF, Liu L, Zhao R. The Ca2+ channel β subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J Gen Physiol 2009; 134: 369–84; PMID:19858357; http://dx.doi.org/ 10.1085/jgp.200910203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suh BC, Kim DI, Falkenburger BH, and Hille B. Membrane localized β-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc Natl Acad Sci USA 2012; 109:3161–6; PMID:22308488; http://dx.doi.org/ 10.1073/pnas.1121434109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miranda-Laferte E, Ewers D, Guzman RE, Jordan N, Schmidt S, Hidalgo P. The N-terminal domain tethers the voltage-gated calcium channel β2e-subunit to the plasma membrane via electrostatic and hydrophobic interactions. J Biol Chem 2014; 289:10387–98; PMID:24519939; http://dx.doi.org/ 10.1074/jbc.M113.507244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim DI, Kang M, Kim S, Lee J, Park Y, Chang I, Suh BC. Molecular basis of the membrane interaction of the β2e subunit of voltage-gated Ca2+ channels. Biophys J 2015a; 109:922–35; http://dx.doi.org/ 10.1016/j.bpj.2015.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim DI, Park Y, Jang DJ, Suh BC. Dynamic phospholipid interaction of β2e subunit regulates the gating of voltage-gated Ca2+ channels. J Gen Physiol 2015b; 39:529–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balla T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol Rev 2013; 93:1019–137; PMID:23899561; http://dx.doi.org/ 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu Rev Biophys Biomol Struct 2005; 34:119–51; PMID:15869386; http://dx.doi.org/ 10.1146/annurev.biophys.33.110502.133337 [DOI] [PubMed] [Google Scholar]
- [16].McLaughlin S, and Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 2005; 438:605–11; PMID:16319880; http://dx.doi.org/ 10.1038/nature04398 [DOI] [PubMed] [Google Scholar]
- [17].Li L, Shi X, Guo X, Li H, Xu C. Ionic protein-lipid interaction at the plasma membrane: what can the charge do? Trends Biochem Sci 2014; 39:130–40; PMID:24534649; http://dx.doi.org/ 10.1016/j.tibs.2014.01.002 [DOI] [PubMed] [Google Scholar]
- [18].McLaughlin S, Hangyás-Mihályné G, Zaitseva I, Golebiewska U. Reversible - through calmodulin - electrostatic interactions between basic residues on proteins and acidic lipids in the plasma membrane. Biochem Soc Symp 2005; 72:189–98; PMID:15649142; http://dx.doi.org/ 10.1042/bss0720189 [DOI] [PubMed] [Google Scholar]
- [19].Arbuzova A, Murray D, McLaughlin S. MARCKS, membranes, and calmodulin: kinetics of their interaction. Biochim Biophys Acta 1998; 1376:369–79; PMID:9804991; http://dx.doi.org/ 10.1016/S0304-4157(98)00011-2 [DOI] [PubMed] [Google Scholar]
- [20].Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, Levine T, Kapus A, Grinstein S. Receptor activation alters inner surface potential during phagocytosis. Science 2006; 313:347–51; PMID:16857939; http://dx.doi.org/ 10.1126/science.1129551 [DOI] [PubMed] [Google Scholar]
- [21].Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science 2008; 319:210–3; PMID:18187657; http://dx.doi.org/ 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- [22].Keum D, Baek C, Kim DI, Kweon HJ, Suh BC. Voltage-dependent regulation of CaV2.2 channels by Gq-coupled receptor is facilitated by membrane-localized β subunit. J Gen Physiol 2014; 144:297–309.; PMID:25225550; http://dx.doi.org/ 10.1085/jgp.201411245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature 1991; 351:320–322; PMID:2034276; http://dx.doi.org/ 10.1038/351320a0 [DOI] [PubMed] [Google Scholar]
- [24].Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 2012; 337:727–30; PMID:22722250; http://dx.doi.org/ 10.1126/science.1222483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsubara M, Titani K, Taniguchi H, Hayashi N. Direct involvement of protein myristoylation in myristoylated alanine-rich C kinase substrate (MARCKS)-calmodulin interaction. J Biol Chem 2003; 278:48898–902; PMID:14506265; http://dx.doi.org/ 10.1074/jbc.M305488200 [DOI] [PubMed] [Google Scholar]
- [26].Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol 1999; 293:321–31; PMID:10550212; http://dx.doi.org/ 10.1006/jmbi.1999.3110 [DOI] [PubMed] [Google Scholar]
- [27].Arbuzova A, Schmitz AA, Vergères G. Cross-talk unfolded: MARCKS proteins. Biochem J 2002; 362:1–12; PMID:11829734; http://dx.doi.org/ 10.1042/bj3620001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 2011; 12:14; PMID:21214938; http://dx.doi.org/ 10.1186/1471-2164-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schachter JB, Sromek SM, Nicholas RA, Harden TK. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology 1997; 36:1181–7; PMID:9364473; http://dx.doi.org/ 10.1016/S0028-3908(97)00138-X [DOI] [PubMed] [Google Scholar]
- [30].Kweon HJ, Yu SY, Kim DI, Suh BC. Differential regulation of proton-sensitive ion channels by phospholipids: a comparative study between ASICs and TRPV1. PLoS One 2015; 10:e0122014; PMID:25781982; http://dx.doi.org/ 10.1371/journal.pone.0122014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Taniguchi CM, Tran TT, Kondo T, Luo J, Ueki K, Cantley LC, Kahn CR. Phosphoinositide 3-kinase regulatory subunit p85α suppresses insulin action via positive regulation of PTEN. Proc Natl Acad Sci U S A 2006; 103:12093–7; PMID:16880400; http://dx.doi.org/ 10.1073/pnas.0604628103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jiménez-Rojo N, Sot J, Viguera AR, Collado MI, Torrecillas A, Gómez-Fernández JC, Goñi FM, Alonso A. Membrane permeabilization induced by sphingosine: Effect of Negatively charged Lipids. Biophys J 2014; 105:2577–84; http://dx.doi.org/ 10.1016/j.bpj.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ohmori S, Sakai N, Shirai Y, Yamamoto H, Miyamoto E, Shimizu N, Saito N. Importance of protein kinase C targeting for the phosphorylation of its substrate, myristoylated alanine-rich C-kinase substrate. J Biol Chem 2000; 265:26449–57; http://dx.doi.org/ 10.1074/jbc.M003588200 [DOI] [PubMed] [Google Scholar]
- [34].Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A, Rebecchi M, Scarlata SF, Runnels LW, Prestwich GD, et al.. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem 1996; 271:26186–93 [DOI] [PubMed] [Google Scholar]