TRP channels show remarkable diversity and influence many physiological functions.1 Most TRP channels are Ca2+ permeable, reside in the plasma membrane and mediate Ca2+ influx in response to various stimuli. However, members of the TRPML (mucolipins) subfamily reside in organelles and function as organellar channels. The subfamily includes three members and was established with the identification of TRPML1 as the protein mutated in the lysosomal storage disease (LSD) Mucolipidosis type IV (MLIV). All TRPML channels function as inward rectifying, Ca2+ permeable cation channels and are activated by the organellar lipid PI(3,5)P2. TRPML1 is largely a lysosomal channel and is cleaved by lysosomal cathepsins, probably as an inactivation mechanism. TRPML3 is expressed mostly in early and late endosomes, while TRPML2 is found mainly in recycling endosomes.2
All TRPML channels function in organellar trafficking; nevertheless based on the knockout mouse phenotype, it appears that the roles of TRPML2 and TRPML3 are modest compared to TRPML1. TRPML3, a pH and Na+ sensitive channel, has a role in autophagy,3 although knockout of TRPML3 has no obvious phenotype. The cellular role of TRPML2 is not well understood, but deletion results in a compromised immune response.4 Inactivating mutations of TRPML1 in humans and deletion of TRPML1 in mice5 result in LSD, indicating critical role of TRPML1 in lysosomal functions.
Early studies demonstrated a role for TRPML1 in trafficking of early and late endosomes to and from the lysosomes, and in fusion of lysosomes with autophagosomes.6 Subsequent studies established TRPML1 as a lysosomal Ca2+ release channel with a role in several lysosomal functions, including large particle phagocytosis, membrane repair and lysosomal trafficking to organelles and molecules designated for degradation.6,7
All the forms of lysosomal trafficking discussed above involve the constitutive trafficking pathway. Another important form of membrane trafficking is that associated with regulated exocytosis, such as secretion by acinar cells within exocrine glands, secretion by endocrine cells, and neurotransmitter release. Surprisingly, the role of the TRPML1 and the effect of any LSDs in regulated exocytosis have not been addressed before, although neurodegeneration is a common feature in all LSDs. In a recent study, we examined the role of TRPML1 in several forms of regulated exocytosis: Ca2+-dependent pancreatic exocytosis, cAMP-dependent salivary gland exocytosis, and neuronal exocytosis of glutamate.8 These studies showed that a major function of TRPML1 is to guard against uncontrolled fusion of the lysosomes with other intracellular organelles. The lysosome enlargement and increased lysosomal undigested content observed in LSDs indicate that the lysosomes do not lose their fusogenic potential in these diseases. This was revealed to be of major consequence in secretory cells containing fusogenic secretory granules and synaptosomes. As illustrated in Figure. 1, lysosomes and secretory granules both reside within the apical pole of secretory cells but do not intermix. Deletion of TRPML1 causes pathological fusion of lysosomes with secretory granules to form highly fusogenic hybrid organelles, resulting in uncontrolled exocytosis.8 This leads to premature activation of digestive enzymes within the pancreatic acinar cells causing chronic pancreatitis. In the neuronal system, excessive exocytosis results in tonic elevation of glutamate, which may contribute to the neurotoxicity in LSDs.8 These findings reveal an unexpected role of TRPML1 in lysosomal function and raise the possibility of using glutamate receptor antagonists, which are widely used for Alzheimer's disease, in the treatment of the neurodegeneration of MLIV.
Figure 1.
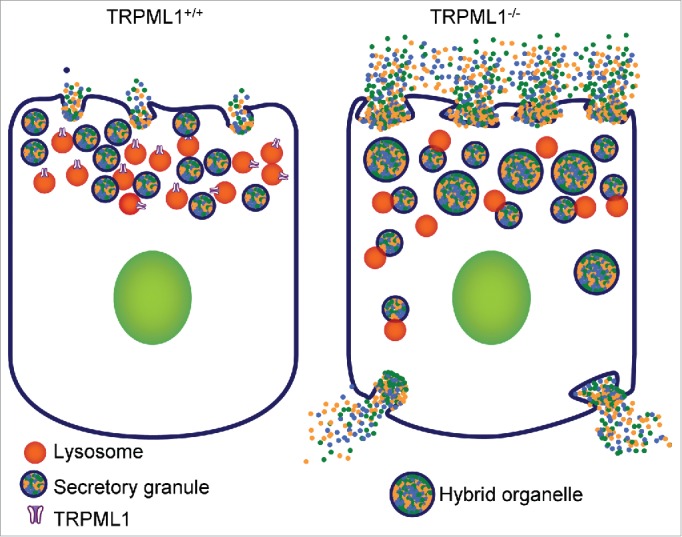
Runaway exocytosis in secretory Trpml1−/− cells. Left panel: in normal cells, the fusogenic secretory granules localize in distinct cellular domains, such as the apical pole in acinar cells, which are also enriched in lysosomes. The pathological fusion between the two organelles is prevented by TRPML1, which controls lysosomal Ca2+ signaling. Right panel: in the absence of TRPML1 or mutations that inhibit TRPML1 function as in MLIV, the lysosomes interact with and fuse with secretory granules to form hybrid organelles. Enhanced fusion of the fusogenic hybrid organelles with the apical membrane results in runaway exocytosis. Part of the hybrid organelles mislocalize and fuse with the basolateral membrane. In addition, the lysosomal proteases cleave the pro-digestive enzymes to prematurely activate them in the hybrid organelles while still in the cells. The combined effects results in pathological cell death as occur in pancreatitis and neurodegeneration.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Comprehensive Physiol 2012; 2:563-608 [DOI] [PubMed] [Google Scholar]
- [2].Xu H, Ren D. Lysosomal physiology. Annual Rev Physiol 2015; 77:57-80; PMID:25668017; http://dx.doi.org/ 10.1146/annurev-physiol-021014-071649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic 2009; 10:1157-67; PMID:19522758; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sun L, Hua Y, Vergarajauregui S, Diab HI, Puertollano R. Novel role of TRPML2 in the regulation of the innate immune response. J Immunol 2015; 195:4922-32; PMID:26432893; http://dx.doi.org/ 10.4049/jimmunol.1500163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chandra M, Zhou H, Li Q, Muallem S, Hofmann SL, Soyombo AA. A role for the Ca2+ channel TRPML1 in gastric acid secretion, based on analysis of knockout mice. Gastroenterology 2011; 140:857-67; PMID:21111738; http://dx.doi.org/ 10.1053/j.gastro.2010.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Venkatachalam K, Wong CO, Zhu MX. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 2015; 58:48-56; PMID:25465891; http://dx.doi.org/ 10.1016/j.ceca.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 2016; (4):404-17; http://dx.doi.org/ 10.1038/ncb3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park S, Ahuja M, Kim MS, Brailoiu GC, Jha A, Zeng M, Baydyuk M, Wu LG, Wassif CA, Porter FD, et al.. Fusion of lysosomes with secretory organelles leads to uncontrolled exocytosis in the lysosomal storage disease mucolipidosis type IV. EMBO Reports 2016; 17:266-78; PMID:26682800; http://dx.doi.org/ 10.15252/embr.201541542 [DOI] [PMC free article] [PubMed] [Google Scholar]


