Water regulation by astroglia is of pivotal significance for the function of the vertebrate CNS. Failure of astrocytic ion/water homeostatic mechanisms may result in irreversible metabolic injury in a wide range of brain pathologies that include water intoxication, liver disease, epilepsy, diabetes, stroke, neurodegenerative disorders and mechanical trauma. The cellular mechanisms that lead to neuronal damage are linked by excessive astroglial swelling caused by osmotically-driven water entry and may result in: (i) cytotoxic edema caused by uncontrolled intracellular accumulation of cations, (ii) compression of blood vessels and neurons by swollen glia, and (iii) attendant reduction in the extracellular volume fraction and excitotoxic elevation of the neurotransmitter glutamate. Treating the initial stages that lead toward glial edema and swelling would seem to be an obvious therapeutic strategy but currently represents a largely intractable challenge for neurosurgeons. Accumulation of excess water in astroglia occurs through aquaporin 4 (AQP4), small (˜30 kDa) integral membrane channel proteins that bidirectionally and selectively transport water in response to osmotic gradients.1 AQP4−/− mice are protected from certain types of mechanical stress including pathological water accumulation in edema yet AQP4 may not always be an appropriate target given that loss-of-function mutations and AQP4 ablation increase susceptibility to seizures.1 Our recent study in retinal glial cells suggests an alternative might be to instead target the mechanism that transduces swelling into downstream cation signals.2 Specifically, we identified the reciprocal interaction between a nonselective cation channel, TRPV4 (transient receptor potential isoform 4) and AQP4 as the key to astroglial swelling, volume regulation and reorganization of downstream signaling pathways in retinal Muller cells. Importantly, we were able to control swelling by targeting either AQP4 or TRPV4 channels.
The study was conducted using antibody staining, gene expression, whole cell recording, calcium imaging and volume measurements in wild type, TRPV4−/− and AQP4−/− Müller cells and in Xenopus oocytes heterologously transfected with TRPV4, AQP4 and TRPV4 & AQP4 genes.2 This comprehensive approach allowed analysis of glial osmosignaling mechanisms under both physiological conditions and during tight genetic and biochemical control of the cellular environment. Müller glia are the principal glial cell type of the vertebrate retina, with radial processes that ensheath neuronal perikarya and synapses and form part of the blood-retinal barrier through endfeet lining inner retinal vasculature. Their many functions: neurotransmitter uptake, structural stabilization, metabolic homeostasis and maintenance of the blood-retina barrier are similar to those performed by brain astrocytes and are essential for retinal survival, however, inflammatory responses mediated by reactive Müller cells can exacerbate retinal injury.3 TRPV4 was found to colocalize with AQP4 and Kir4.1 subunits in glial endfeet, suggesting a novel mechanism for transducing osmotic stress at the blood-retina barrier.2 TRPV4−/− and AQP4−/− retinas did not exhibit a phenotype in terms of morphology, protein trafficking or transcription of housekeeping genes whereas TRPV4-deficient retinas showed mild levels of reactive gliosis, consistent with the importance of the channel in glial steady-state function.4,5 Interestingly, elimination of TRPV4 was associated with reduced mRNA levels of Aqp4 and Kcnj10 (which encodes the Kir4.1 inward-rectifying K+ channel) whereas AQP4−/− retinas showed suppressed Kcnj10 transcription. The interdependence of 3 highly dissimilar osmo-relevant channels at the molecular level might reflect clustering with the Müller transcriptome or linkage through activity- and/or TRPV4-dependent Ca2+ entry.
Elimination of AQP4 slowed the rate of glial swelling and dramatically reduced the amplitude of hypotonically induced cation currents and [Ca2+]i elevations. Given that AQP4 is impermeable to ions, its activation must be coupled to a Ca2+-permeable cation channel - TRPV4. We discovered that the interaction between TRPV4 and AQP4 in Müller cells is reciprocal: AQP4-dependent currents/[Ca2+]i elevations and swelling were inhibited by selective TRPV4 blockers whereas AQP4 ablation reduced both TRPV4-mediated Ca2+ influx and the rate of cellular volume increase. To better define the mechanism, we turned to the Xenopus oocyte expression model. AQP4 and TRPV4 channels were required for oocyte swelling and cation influx, respectively, however, coexpression dramatically augmented cells' sensitivity to osmotic stress by allowing cation influx at much smaller osmotic gradients. When the swelling rate was osmotically matched for AQP4- positive and negative cells, TRPV4 activation became independent of AQP4. This suggests that AQP4 functions as an elegant molecular amplification device that transduces osmotic stress into long-term response through changes in [Ca2+]i.2 Sustained over time, this may result in reactive gliosis and inflammation.5 A similar conclusion was concurrently reached for cortical astroglia.7
The study also addressed the potential contribution of TRPV4-AQP4 interactions to regulatory volume decrease (RVD), a key process that protects cells against pathological swelling. Its precise mechanism is cell type-dependent but was suggested to require TRPV4 and AQP4 in cortical astrocytes.6 We found that AQP4-mediated facilitation of Ca2+ influx through TRPV4 channels facilitates Müller cell RVD yet this was observed only under conditions that are unlikely to occur in vivo (i.e., ∼50% reduction in tonicity). In contrast, TRPV4 activation (by agonists or swelling) itself augmented glial swelling through an unknown mechanism that required an increase in [Ca2+]i and, possibly, activation of phospholipase A2.2,4,5
We hypothesize that coordination of activity-dependent ionic/water fluxes at the blood-retina barrier critically depends on functional interactions between endfoot TRPV4, AQP4 and Kir4.1 channels. This osmoregulatory complex links changes in glial water permeability, K+ uptake, ATP release and Ca2+ homeostasis that occur in response to during neuronal firing,1,8 to downstream modulation of Aqp4 and Kcnj10 and astroglial function by maintaining the steady-state ‘osmo-tensile’ homeostasis. In the presence of prolonged osmotic stress or pathological circumstances (such as diabetes, traumatic ocular injury or glaucoma), however, TRPV4-AQP4 interactions associated with excessive swelling of CNS astroglia ‘turbo-charge’ inflammatory remodeling through deranged Ca2+ signaling, reactive gliosis and macular or brain edema.1,2,4,5,8
Figure 1.
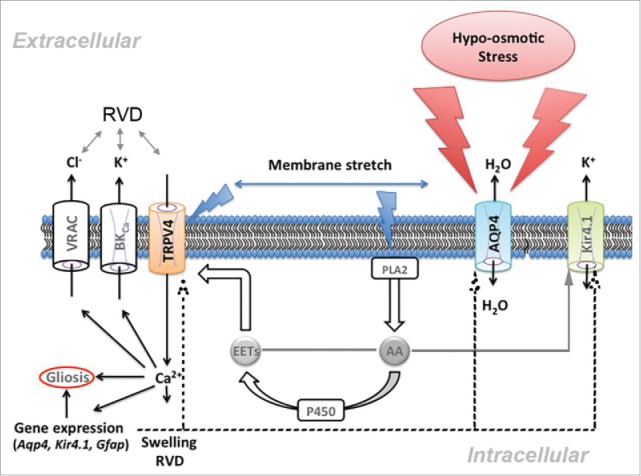
Functional interactions between TRPV4 and AQP4 channels regulate glial physiology. Water influx through AQP4 stretches the Müller cell plasma membrane, activating TRPV4 via stretch-sensitive phospholipase A2 (PLA2) and eicosanoid messengers (EETs). The subsequent increase in [Ca2+]i regulates osmorelevant (Aqp4, Kir4.1/Kcnj10, Trpv4) and proinflammatory (Gfap) genes and facilitates cell swelling. High [Ca2+]i may stimulate BK (Ca2+-dependent K+) and VRAC (volume-activated anion) channels, induce RVD and facilitate reactive gliosis. Further details in Jo et al. (2015).2
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov 2014; 13(4):259-77; PMID:24625825; http://dx.doi.org/ 10.1038/nrd4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jo AO, Ryskamp DA, Phuong TT, Verkman AS, Yarishkin O, MacAulay N, Križaj D. TRPV4 and AQP4 Channels Synergistically Regulate Cell Volume and Calcium Homeostasis in Retinal Müller Glia. J Neurosci 2015; 35(39):13525-37; PMID:26424896; http://dx.doi.org/ 10.1523/JNEUROSCI.1987-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reichenbach A, Bringmann A. New functions of Müller cells. Glia 2013; 61(5):651-78; PMID:23440929; http://dx.doi.org/ 10.1002/glia.22477 [DOI] [PubMed] [Google Scholar]
- [4].Ryskamp DA, Iuso A, Križaj D. TRPV4 links inflammatory signaling and neuroglial swelling. Channels 2015; 9(2):70-2; http://dx.doi.org/ 10.1080/19336950.2015.1017998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, Križaj D. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci 2014; 34:15689-700; PMID:25411497; http://dx.doi.org/ 10.1523/JNEUROSCI.2540-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A 2011; 108:2563-8; http://dx.doi.org/ 10.1073/pnas.1012867108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mola MG, Sparaneo A, Gargano CD, Spray DC, Svelto M, Frigeri A, Scemes E, Nicchia GP. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins. Glia 2016 Jan;64(1):139-5; PMID:26413835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Thrane VR, Enger R, et al.. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A 2011; 108:846-51; PMID:21187412; http://dx.doi.org/ 10.1073/pnas.1015217108 [DOI] [PMC free article] [PubMed] [Google Scholar]


